Haptoglobin Treatment for Aneurysmal Subarachnoid Hemorrhage: Review and Expert Consensus on Clinical Translation
- PMID: 37232189
- PMCID: PMC10289236
- DOI: 10.1161/STROKEAHA.123.040205
Haptoglobin Treatment for Aneurysmal Subarachnoid Hemorrhage: Review and Expert Consensus on Clinical Translation
Abstract
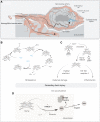
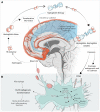
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating form of stroke frequently affecting young to middle-aged adults, with an unmet need to improve outcome. This special report focusses on the development of intrathecal haptoglobin supplementation as a treatment by reviewing current knowledge and progress, arriving at a Delphi-based global consensus regarding the pathophysiological role of extracellular hemoglobin and research priorities for clinical translation of hemoglobin-scavenging therapeutics. After aneurysmal subarachnoid hemorrhage, erythrocyte lysis generates cell-free hemoglobin in the cerebrospinal fluid, which is a strong determinant of secondary brain injury and long-term clinical outcome. Haptoglobin is the body's first-line defense against cell-free hemoglobin by binding it irreversibly, preventing translocation of hemoglobin into the brain parenchyma and nitric oxide-sensitive functional compartments of cerebral arteries. In mouse and sheep models, intraventricular administration of haptoglobin reversed hemoglobin-induced clinical, histological, and biochemical features of human aneurysmal subarachnoid hemorrhage. Clinical translation of this strategy imposes unique challenges set by the novel mode of action and the anticipated need for intrathecal drug administration, necessitating early input from stakeholders. Practising clinicians (n=72) and scientific experts (n=28) from 5 continents participated in the Delphi study. Inflammation, microvascular spasm, initial intracranial pressure increase, and disruption of nitric oxide signaling were deemed the most important pathophysiological pathways determining outcome. Cell-free hemoglobin was thought to play an important role mostly in pathways related to iron toxicity, oxidative stress, nitric oxide, and inflammation. While useful, there was consensus that further preclinical work was not a priority, with most believing the field was ready for an early phase trial. The highest research priorities were related to confirming haptoglobin's anticipated safety, individualized versus standard dosing, timing of treatment, pharmacokinetics, pharmacodynamics, and outcome measure selection. These results highlight the need for early phase trials of intracranial haptoglobin for aneurysmal subarachnoid hemorrhage, and the value of early input from clinical disciplines on a global scale during the early stages of clinical translation.
Keywords: blood; haptoglobins; hemoglobins; subarachnoid hemorrhage; therapeutics.
Conflict of interest statement
Figures



References
-
- Etminan N, Chang H-S, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population. Jama Neurol. 2019;76:588–597. doi: 10.1001/jamaneurol.2019.0006 - PMC - PubMed
-
- Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459 - PubMed
-
- Chou SH-Y. Subarachnoid hemorrhage. Continuum. 2021;27:1201–1245. doi: 10.1212/CON.0000000000001052 - PubMed
-
- Khan AU, Dulhanty L, Vail A, Tyrrell P, Galea J, Patel HC. Impact of specialist neurovascular care in subarachnoid haemorrhage. Clin Neurol Neurosurg. 2015;133:55–60. doi: 10.1016/j.clineuro.2015.03.006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

