A Randomized Controlled Trial to Compare Immunogenicity to Cell-Based Versus Live-Attenuated Influenza Vaccines in Children
- PMID: 37232430
- PMCID: PMC10312301
- DOI: 10.1093/jpids/piad033
A Randomized Controlled Trial to Compare Immunogenicity to Cell-Based Versus Live-Attenuated Influenza Vaccines in Children
Abstract
Background: Few studies have focused on the immune response to more recent influenza vaccine formulations such as cell-cultured inactivated influenza vaccine (ccIIV4) or live-attenuated influenza vaccine (LAIV4) in older children and young adults, or differences in immunoglobulin response using newer antibody landscape technology.
Methods: Participants ages 4-21 were randomized to receive ccIIV4 (n = 112) or LAIV4 (n = 118). A novel high-throughput multiplex influenza antibody detection assay was used to provide detailed IgG, IgA, and IgM antibody isotypes, along with hemagglutination inhibition levels (HAI), measured pre- and 28 days post-vaccination.
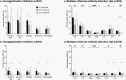
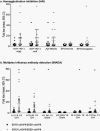
Results: The HAI and immunoglobulin isotype response to ccIIV4 was greater than LAIV4, with significant increases in IgG but not IgA or IgM. The youngest participants had the highest LAIV4 response. Prior LAIV4 vaccination was associated with a higher response to current season ccIIV4. Cross-reactive A/Delaware/55/2019(H1N1)pdm09 antibodies were present pre-vaccination and increased in response to ccIIV4, but not LAIV4. Immunoglobulin assays strongly correlated with and confirmed the findings of HAI titers to measure immune response.
Conclusions: Age and prior season vaccination may play a role in the immune response in children and young adults to ccIIV4 and LAIV4. While immunoglobulin isotypes provide high-level antigen-specific information, HAI titers alone can provide a meaningful representation of day 28 post-vaccination response.
Clinical trials no: NCT03982069.
Keywords: MIADA; cell-culture-based inactivated influenza vaccine; egg-based live-attenuated influenza vaccine; hemagglutination inhibition assay; influenza; influenza vaccine; randomized controlled trial.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2021–22 . https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendatio.... Accessed September 22, 2021.
-
- Centers for Disease Control. Disease burden of influenza . https://www.cdc.gov/flu/about/burden/index.html. Accessed June 30, 2021.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

