Temperature-Promoted Giant Unilamellar Vesicle (GUV) Aggregation: A Way of Multicellular Formation
- PMID: 37232711
- PMCID: PMC10217545
- DOI: 10.3390/cimb45050242
Temperature-Promoted Giant Unilamellar Vesicle (GUV) Aggregation: A Way of Multicellular Formation
Abstract
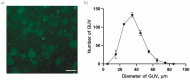
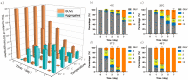
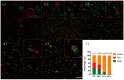
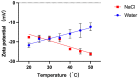
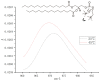
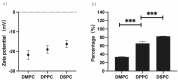
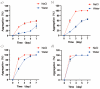
The evolution of unicellular to multicellular life is considered to be an important step in the origin of life, and it is crucial to study the influence of environmental factors on this process through cell models in the laboratory. In this paper, we used giant unilamellar vesicles (GUVs) as a cell model to investigate the relationship between environmental temperature changes and the evolution of unicellular to multicellular life. The zeta potential of GUVs and the conformation of the headgroup of phospholipid molecules at different temperatures were examined using phase analysis light scattering (PALS) and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR), respectively. In addition, the effect of increasing temperature on the aggregation of GUVs was further investigated in ionic solutions, and the possible mechanisms involved were explored. The results showed that increasing temperature reduced the repulsive forces between cells models and promoted their aggregation. This study could effectively contribute to our understanding of the evolution of primitive unicellular to multicellular life.
Keywords: aggregation; giant unilamellar vesicles; headgroup reorientation; phospholipid; zeta potential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Clustering of Giant Unilamellar Vesicles Promoted by Covalent and Noncovalent Bonding of Functional Groups at Membrane-Embedded Peptides.Bioconjug Chem. 2019 Aug 21;30(8):2156-2164. doi: 10.1021/acs.bioconjchem.9b00394. Epub 2019 Aug 7. Bioconjug Chem. 2019. PMID: 31322865
-
Antimicrobial peptide magainin 2-induced rupture of single giant unilamellar vesicles comprising E. coli polar lipids.Biochim Biophys Acta Biomembr. 2023 Mar;1865(3):184112. doi: 10.1016/j.bbamem.2022.184112. Epub 2022 Dec 22. Biochim Biophys Acta Biomembr. 2023. PMID: 36567034
-
Aggregation Control of Gold Nanoparticles and Surface-Enhanced Raman Scattering within Giant Unilamellar Vesicles.Langmuir. 2025 Apr 15;41(14):9567-9573. doi: 10.1021/acs.langmuir.5c00730. Epub 2025 Mar 31. Langmuir. 2025. PMID: 40163099
-
Reconstitution of an RNA Virus Replicase in Artificial Giant Unilamellar Vesicles Supports Full Replication and Provides Protection for the Double-Stranded RNA Replication Intermediate.J Virol. 2020 Aug 31;94(18):e00267-20. doi: 10.1128/JVI.00267-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641477 Free PMC article.
-
Giant unilamellar vesicles - a perfect tool to visualize phase separation and lipid rafts in model systems.Acta Biochim Pol. 2009;56(1):33-9. Epub 2009 Mar 17. Acta Biochim Pol. 2009. PMID: 19287805 Review.
Cited by
-
Synthetic Cells Revisited: Artificial Cells Construction Using Polymeric Building Blocks.Adv Sci (Weinh). 2024 Feb;11(8):e2305837. doi: 10.1002/advs.202305837. Epub 2023 Nov 20. Adv Sci (Weinh). 2024. PMID: 37984885 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

