Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods
- PMID: 37232716
- PMCID: PMC10217460
- DOI: 10.3390/cimb45050247
Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods
Abstract
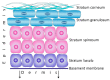
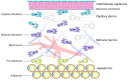
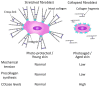
This article includes the data from current studies regarding the pathophysiological mechanisms of skin aging and the regenerative processes occurring in the epidermis and dermis at the molecular and cellular level, mainly, the key role of dermal fibroblasts in skin regeneration. Analyzing these data, the authors proposed the concept of skin anti-age therapy that is based on the correction of age-related skin changes by stimulating regenerative processes at the molecular and cellular level. The main target of the skin anti-age therapy is dermal fibroblasts (DFs). A variant of the cosmetological anti-age program using the combination of laser and cellular methods of regenerative medicine is presented in the paper. The program includes three stages of implementation and defines the tasks and methods of each stage. Thus, laser technologies allow one to remodel the collagen matrix and create favorable conditions for DFs functions, whereas the cultivated autologous dermal fibroblasts replenish the pool of mature DFs decreasing with age and are responsible for the synthesis of components of the dermal extracellular matrix. Finally, the use of autological platelet-rich plasma (PRP) enables to maintenance of the achieved results by stimulating DF function. It has been shown that growth factors/cytokines contained in α-granules of platelets injected into the skin bind to the corresponding transmembrane receptors on the surface of DFs and stimulate their synthetic activity. Thus, the consecutive, step-by-step application of the described methods of regenerative medicine amplifies the effect on the molecular and cellular aging processes and thereby allows one to optimize and prolong the clinical results of skin rejuvenation.
Keywords: PRP therapy; cell therapy; correction of age-related skin changes; laser therapy; skin fibroblasts; skin structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

