Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells
- PMID: 37232726
- PMCID: PMC10217017
- DOI: 10.3390/cimb45050257
Anti-Leukemic Effects of Idesia polycarpa Maxim Branch on Human B-Cell Acute Lymphoblastic Leukemia Cells
Abstract
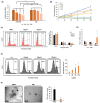
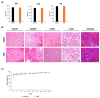
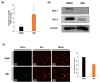
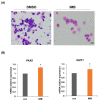
Patients with pediatric B-cell acute lymphoblastic leukemia (B-ALL) have a high survival rate, yet the prognosis of adults and patients with relapsed/refractory disease is relatively poor. Therefore, it is imperative to develop new therapeutic strategies. Here, we screened 100 plant extracts from South Korean Flora and investigated their anti-leukemic effect using CCRF-SB cells as a B-ALL model. The top cytotoxic extract identified in this screening was the Idesia polycarpa Maxim. branch (IMB), which efficiently inhibited the survival and proliferation of CCRF-SB cells, while having minimal to no impact on normal murine bone marrow cells. Mechanistically, the IMB-induced proapoptotic effect involves the increase of caspase 3/7 activity, which was shown to be associated with the disruption of the mitochondrial membrane potential (MMP) through the reduction in antiapoptotic Bcl-2 family expression. IMB also promoted the differentiation of CCRF-SB cells via the upregulation of the expression of differentiation-related genes, PAX5 and IKZF1. Given that resistance to glucocorticoid (GC) is often found in patients with relapsed/refractory ALL, we investigated whether IMB could restore GC sensitivity. IMB synergized GC to enhance apoptotic rate by increasing GC receptor expression and downmodulating mTOR and MAPK signals in CCRF-SB B-ALL cells. These results suggest that IMB has the potential to be a novel candidate for the treatment of B-ALL.
Keywords: B-cell acute lymphoblastic leukemia; Idesia polycarpa Maxim branch; anti-leukemic effect; differentiation; glucocorticoid resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The development and application of compound formulation of natural antioxidants in Idesia polycarpa maxim. Oil.Heliyon. 2025 Jan 3;11(1):e41648. doi: 10.1016/j.heliyon.2025.e41648. eCollection 2025 Jan 15. Heliyon. 2025. PMID: 39866486 Free PMC article.
-
Evaluation of the use of Idesia polycarpa Maxim protein coating to extend the shelf life of European sweet cherries.Front Nutr. 2023 Nov 17;10:1283086. doi: 10.3389/fnut.2023.1283086. eCollection 2023. Front Nutr. 2023. PMID: 38045816 Free PMC article.
-
Cytotoxic and apoptotic effects of prenylflavonoid artonin B in human acute lymphoblastic leukemia cells.Acta Pharmacol Sin. 2006 Sep;27(9):1165-74. doi: 10.1111/j.1745-7254.2006.00404.x. Acta Pharmacol Sin. 2006. PMID: 16923337
-
Separation and purification of antioxidant peptides from Idesia polycarpa Maxim. cake meal and study of conformational relationship between them.Food Sci Nutr. 2024 Jul 10;12(9):6206-6225. doi: 10.1002/fsn3.4325. eCollection 2024 Sep. Food Sci Nutr. 2024. PMID: 39554371 Free PMC article. Review.
-
Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol.Front Oncol. 2021 Mar 11;11:617937. doi: 10.3389/fonc.2021.617937. eCollection 2021. Front Oncol. 2021. PMID: 33777761 Free PMC article. Review.
Cited by
-
Genetic Diversity Analysis and Polyploid Induction Identification of Idesia polycarpa.Plants (Basel). 2024 Dec 3;13(23):3394. doi: 10.3390/plants13233394. Plants (Basel). 2024. PMID: 39683187 Free PMC article.
-
Norchelerythrine from Corydalis incisa (Thunb.) Pers. promotes differentiation and apoptosis by activating DNA damage response in acute myeloid leukemia.Int J Oncol. 2025 Mar;66(3):17. doi: 10.3892/ijo.2025.5723. Epub 2025 Feb 7. Int J Oncol. 2025. PMID: 39918000 Free PMC article.
-
Ajania pacifica (Nakai) K. Bremer and Humphries Extract Limits MYC Expression to Induce Apoptosis in Diffuse Large B Cell Lymphoma.Curr Issues Mol Biol. 2024 May 11;46(5):4580-4594. doi: 10.3390/cimb46050278. Curr Issues Mol Biol. 2024. PMID: 38785546 Free PMC article.
-
Therapeutic Potential of Adina rubella Hance Stem and Picroside III as a Differentiation Inducer in AML Cells via Mitochondrial ROS Accumulation.Int J Mol Sci. 2025 Feb 5;26(3):1350. doi: 10.3390/ijms26031350. Int J Mol Sci. 2025. PMID: 39941121 Free PMC article.
References
-
- Schrappe M., Reiter A., Ludwig W.D., Harbott J., Zimmermann M., Hiddemann W., Niemeyer C., Henze G., Feldges A., Zintl F., et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: Results of trial ALL-BFM 90. Blood. 2000;95:3310–3322. - PubMed
-
- Boissel N., Auclerc M.F., Lhéritier V., Perel Y., Thomas X., Leblanc T., Rousselot P., Cayuela J.M., Gabert J., Fegueux N., et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J. Clin. Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. - DOI - PubMed
-
- Annino L., Vegna M.L., Camera A., Specchia G., Visani G., Fioritoni G., Ferrara F., Peta A., Ciolli S., Deplano W., et al. Treatment of adult acute lymphoblastic leukemia (ALL): Long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.V99.3.863. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

