Is Th17-Targeted Therapy Effective in Systemic Lupus Erythematosus?
- PMID: 37232744
- PMCID: PMC10217373
- DOI: 10.3390/cimb45050275
Is Th17-Targeted Therapy Effective in Systemic Lupus Erythematosus?
Abstract
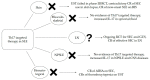
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with a broad spectrum of clinical manifestations. The proposed pathophysiological hypotheses of SLE are numerous, involving both innate and adaptive abnormal immune responses. SLE is characterized by the overproduction of different autoantibodies that form immune complexes, which cause damage in different organs. Current therapeutic modalities are anti-inflammatory and immunosuppressive. In the last decade, we have witnessed the development of many biologicals targeting different cytokines and other molecules. One of them is interleukin-17 (IL-17), a central cytokine of a proinflammatory process that is mediated by a group of helper T cells called Th17. Direct inhibitors of IL-17 are used in psoriatic arthritis, spondyloarthritis, and other diseases. Evidence about the therapeutic potential of Th17-targeted therapies in SLE is scarce, and probably the most promising is related to lupus nephritis. As SLE is a complex heterogeneous disease with different cytokines involved in its pathogenesis, it is highly unlikely that inhibition of only one molecule, such as IL-17, will be effective in the treatment of all clinical manifestations. Future studies should identify SLE patients that are eligible for Th17-targeted therapy.
Keywords: guselkumab; interleukin-17; interleukin-23; lupus nephritis; secukinumab; systemic lupus erythematosus; ustekinumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Papp K., Végh P., Hóbor R., Szittner Z., Vokó Z., Podani J., Czirják L., Prechl J. Immune complex signatures of patients with active and inactive SLE revealed by multiplex protein bindin analysis on antigen microarrays. PLoS ONE. 2012;7:e44824. doi: 10.1371/journal.pone.0044824. - DOI - PMC - PubMed
-
- Aringer M., Costenbader K., Daikh D., Brinks R., Mosca M., Ramsey-Goldman R., Smolen J.S., Wofsy D., Boumpas D.T., Kamen D.L., et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019;78:1151–1159. doi: 10.1136/annrheumdis-2018-214819. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

