Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients
- PMID: 37232793
- PMCID: PMC10217267
- DOI: 10.3390/curroncol30050335
Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients
Abstract
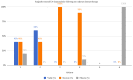
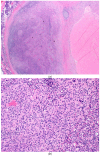
Background: Early-phase neoadjuvant trials have demonstrated promising results in the utility of upfront immunotherapy in locally advanced stage III melanoma and unresected nodal disease. Secondary to these results and the COVID-19 pandemic, this patient population, traditionally managed through surgical resection and adjuvant immunotherapy, received a novel treatment strategy of neoadjuvant therapy (NAT). Methods: Patients with node-positive disease, who faced surgical delays secondary to COVID-19, were treated with NAT, followed by surgery. Demographic, tumour, treatment and response data were collected through a retrospective chart review. Biopsy specimens were analysed prior to the initiation of NAT, and therapy response was analysed following surgical resection. NAT tolerability was recorded. Results: Six patients were included in this case series; four were treated with nivolumab alone, one with ipilimumab and nivolumab and one with dabrafenib and trametinib. Twenty-two incidents of adverse events were reported, with the majority (90.9%) being classified as grade one or two. All patients underwent surgical resection: three out of six patients following two NAT cycles, two following three cycles and one following six cycles. Surgically resected samples were histopathologically evaluated for the presence of disease. Five out of six patients (83%) had ≤1 positive lymph node. One patient showed extracapsular extension. Four patients demonstrated complete pathological response; two had persisting viable tumour cells. Conclusions: In this case series, we outlined how in response to surgical delays secondary to the COVID-19 pandemic, NAT was successfully applied to achieve promising treatment response in patients with locally advanced stage III melanoma.
Keywords: COVID-19; melanoma; neoadjuvant therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Canadian Institute for Health Information COVID-19′s Impact on Hospital Services. [(accessed on 11 March 2023)]. Available online: https://www.cihi.ca/en/covid-19-resources/impact-of-covid-19-on-canadas-....
-
- A Ascierto P., Del Vecchio M., Mandalá M., Gogas H., Arance A.M., Dalle S., Cowey C.L., Schenker M., Grob J.-J., Chiarion-Sileni V., et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:1465–1477. doi: 10.1016/S1470-2045(20)30494-0. - DOI - PubMed
-
- Eggermont A.M.M., Blank C.U., Mandalà M., Long G.V., Atkinson V.G., Dalle S., Haydon A.M., Meshcheryakov A., Khattak A., Carlino M.S., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:643–654. doi: 10.1016/S1470-2045(21)00065-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

