In-Situ Fabrication of Electroactive Cu2+-Trithiocyanate Complex and Its Application for Label-Free Electrochemical Aptasensing of Thrombin
- PMID: 37232893
- PMCID: PMC10216145
- DOI: 10.3390/bios13050532
In-Situ Fabrication of Electroactive Cu2+-Trithiocyanate Complex and Its Application for Label-Free Electrochemical Aptasensing of Thrombin
Abstract
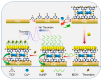
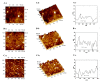
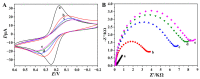
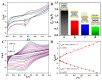
The preparation of an electroactive matrix for the immobilization of the bioprobe shows great promise to construct the label-free biosensors. Herein, the electroactive metal-organic coordination polymer has been in-situ prepared by pre-assembly of a layer of trithiocynate (TCY) on a gold electrode (AuE) through Au-S bond, followed by repetitive soaking in Cu(NO3)2 solution and TCY solutions. Then the gold nanoparticles (AuNPs) and the thiolated thrombin aptamers were successively assembled on the electrode surface, and thus the electrochemical electroactive aptasensing layer for thrombin was achieved. The preparation process of the biosensor was characterized by an atomic force microscope (AFM), attenuated total reflection-Fourier transform infrared (ATR-FTIR), and electrochemical methods. Electrochemical sensing assays showed that the formation of the aptamer-thrombin complex changed the microenvironment and the electro-conductivity of the electrode interface, causing the electrochemical signal suppression of the TCY-Cu2+ polymer. Additionally, the target thrombin can be label-free analyzed. Under optimal conditions, the aptasensor can detect thrombin in the concentration range from 1.0 fM to 1.0 μM, with a detection limit of 0.26 fM. The spiked recovery assay showed that the recovery of the thrombin in human serum samples was 97.2-103%, showing that the biosensor is feasible for biomolecule analysis in a complex sample.
Keywords: Cu2+; aptasensor; electrochemical; thrombin; trithiocynate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Label-free electrochemical aptasensor for sensitive thrombin detection using layer-by-layer self-assembled multilayers with toluidine blue-graphene composites and gold nanoparticles.Talanta. 2012 Aug 30;98:7-13. doi: 10.1016/j.talanta.2012.06.019. Epub 2012 Jun 22. Talanta. 2012. PMID: 22939121
-
Highly sensitive electrochemical label-free aptasensor based on dual electrocatalytic amplification of Pt-AuNPs and HRP.Analyst. 2011 May 7;136(9):1840-5. doi: 10.1039/c0an00755b. Epub 2011 Mar 7. Analyst. 2011. PMID: 21380419
-
Label-free electrochemical detection of human α-thrombin in blood serum using ferrocene-coated gold nanoparticles.Biosens Bioelectron. 2011 Oct 15;28(1):454-8. doi: 10.1016/j.bios.2011.06.040. Epub 2011 Jul 6. Biosens Bioelectron. 2011. PMID: 21802275
-
Role of Estradiol Hormone in Human Life and Electrochemical Aptasensing of 17β-Estradiol: A Review.Biosensors (Basel). 2022 Dec 2;12(12):1117. doi: 10.3390/bios12121117. Biosensors (Basel). 2022. PMID: 36551086 Free PMC article. Review.
-
Nucleic acid aptamer-based biosensors and their application in thrombin analysis.Bioanalysis. 2023 May;15(9):513-532. doi: 10.4155/bio-2023-0058. Epub 2023 Jun 16. Bioanalysis. 2023. PMID: 37326345 Review.
References
-
- Zhu Y., Qi Y., Xu M., Luo J. Flexible biosensor based on signal amplification of gold nanoparticles-composite flower clusters for glucose detection in sweat. Colloids Surf. A Physicochem. Eng. Asp. 2023;661:130908. doi: 10.1016/j.colsurfa.2022.130908. - DOI
-
- Sumitha M., Xavier T. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023;2:100023. doi: 10.1016/j.hybadv.2023.100023. - DOI
-
- Lakshmi G., Poddar M., Dhiman T.K., Singh A.K., Solanki P.R. Gold-Ceria nanocomposite based highly sensitive and selective aptasensing platform for the detection of the Chlorpyrifos in Solanum tuberosum. Colloids Surf. A Physicochem. Eng. Asp. 2022;653:129819. doi: 10.1016/j.colsurfa.2022.129819. - DOI
-
- Hasan M.R., Sharma P., Shaikh S., Singh S., Pilloton R., Narang J. Electrochemical Aptasensor Developed Using Two-Electrode Setup and Three-Electrode Setup: Comprising Their Current Range in Context of Dengue Virus Determination. Biosensors. 2022;13:1. doi: 10.3390/bios13010001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

