Transcriptome Analysis of Co-Cultures of THP-1 Human Macrophages with Inactivated Germinated Trichophyton rubrum Conidia
- PMID: 37233274
- PMCID: PMC10219549
- DOI: 10.3390/jof9050563
Transcriptome Analysis of Co-Cultures of THP-1 Human Macrophages with Inactivated Germinated Trichophyton rubrum Conidia
Abstract
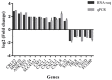
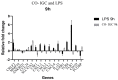
Although most mycoses are superficial, the dermatophyte Trichophyton rubrum can cause systemic infections in patients with a weakened immune system, resulting in serious and deep lesions. The aim of this study was to analyze the transcriptome of a human monocyte/macrophage cell line (THP-1) co-cultured with inactivated germinated T. rubrum conidia (IGC) in order to characterize deep infection. Analysis of macrophage viability by lactate dehydrogenase quantification showed the activation of the immune system after 24 h of contact with live germinated T. rubrum conidia (LGC). After standardization of the co-culture conditions, the release of the interleukins TNF-α, IL-8, and IL-12 was quantified. The greater release of IL-12 was observed during co-culturing of THP-1 with IGC, while there was no change in the other cytokines. Next-generation sequencing of the response to T. rubrum IGC identified the modulation of 83 genes; of these, 65 were induced and 18 were repressed. The categorization of the modulated genes showed their involvement in signal transduction, cell communication, and immune response pathways. In total, 16 genes were selected for validation and Pearson's correlation coefficient was 0.98, indicating a high correlation between RNA-seq and qPCR. Modulation of the expression of all genes was similar for LGC and IGC co-culture; however, the fold-change values were higher for LGC. Due to the high expression of the IL-32 gene in RNA-seq, we quantified this interleukin and observed an increased release in co-culture with T. rubrum. In conclusion, the macrophages-T. rubrum co-culture model revealed the ability of these cells to modulate the immune response, as demonstrated by the release of proinflammatory cytokines and the RNA-seq gene expression profile. The results obtained permit to identify possible molecular targets that are modulated in macrophages and that could be explored in antifungal therapies involving the activation of the immune system.
Keywords: IL-32; LPS; RNA sequencing; deep infection; dermatophytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hay R.J. Hunter’s Tropical Medicine and Emerging Infectious Diseases. Elsevier; Amsterdam, The Netherlands: 2020. Superficial Mycoses; pp. 648–652.
-
- Sai S.B., Tejashree A., Veeranna S., Krishna K.M. Speciation and in vitro activity of four antifungal drugs against clinical isolates of dermatophytes by e-test method. Int. J. Sci. Res. 2019;8:6.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

