Surface Coatings of Dental Implants: A Review
- PMID: 37233397
- PMCID: PMC10218820
- DOI: 10.3390/jfb14050287
Surface Coatings of Dental Implants: A Review
Abstract
Replacement of missing teeth is possible using biocompatible devices such as endosseous implants. This study aims to analyze and recognize the best characteristics of different implant surfaces that ensure good peri-implant tissue healing and thus clinical success over time. The present review was performed on the recent literature concerning endosseous implants made of titanium, a material most frequently used because of its mechanical, physical, and chemical characteristics. Thanks to its low bioactivity, titanium exhibits slow osseointegration. Implant surfaces are treated so that cells do not reject the surface as a foreign material and accept it as fully biocompatible. Analysis of different types of implant surface coatings was performed in order to identify ideal surfaces that improve osseointegration, epithelial attachment to the implant site, and overall peri-implant health. This study shows that the implant surface, with different adhesion, proliferation, and spreading capabilities of osteoblastic and epithelial cells, influences the cells involved in anchorage. Implant surfaces must have antibacterial capabilities to prevent peri-implant disease. Research still needs to improve implant material to minimize clinical failure.
Keywords: bacterial adhesion; coating; dental implant; implant stability; marginal bone level; osseointegration; peri-implant health; surface; titanium; treatment surface.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




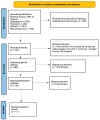
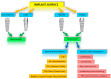


References
-
- Calabriso N., Stanca E., Rochira A., Damiano F., Giannotti L., Di Chiara Stanca B., Massaro M., Scoditti E., Demitri C., Nitti P., et al. Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components. Pharmaceutics. 2021;13:635. doi: 10.3390/pharmaceutics13050635. - DOI - PMC - PubMed
-
- Khurshid Z., Hafeji S., Tekin S., Habib S.R., Ullah R., Sefat F., Zafar M.S. 2—Titanium, Zirconia, and Polyetheretherketone (PEEK) as a Dental Implant Material. In: Zafar M.S., Khurshid Z., Khan A.S., Najeeb S., Sefat F., editors. Dental Implants. Woodhead Publishing; Cambridge, UK: 2020. pp. 5–35. (Woodhead Publishing Series in Biomaterials).
Publication types
LinkOut - more resources
Full Text Sources

