Leucine (and lysine) increased plasma levels of the satiety hormone cholecystokinin (CCK), and phenylalanine of the incretin glucagon-like peptide 1 (GLP-1) after oral gavages in pigs
- PMID: 37233611
- PMCID: PMC10263118
- DOI: 10.1093/jas/skad175
Leucine (and lysine) increased plasma levels of the satiety hormone cholecystokinin (CCK), and phenylalanine of the incretin glucagon-like peptide 1 (GLP-1) after oral gavages in pigs
Abstract
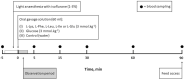
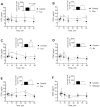
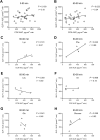
Excess dietary amino acids (AA) has been associated with reduced feed intake, increased satiation, and extended satiety in pigs. Recent ex vivo studies suggested that satiety peptide cholecystokinin (CCK) and insulinotropic glucagon-like peptide 1 (GLP-1), mediated the anorexigenic or insulinotropic effects of Lys, Glu, Phe, Ile, and Leu. However, the ex vivo model limitations require validation in vivo. The aim of the present study was to assess the effect of orally administered AA in vivo in pigs. It was hypothesized that oral Lys, Ile, and Leu have an anorexigenic effect via CCK, while Glu and Phe have an insulinotropic effect increasing circulating levels of GLP-1. Eight entire male pigs (Landrace × Large White) of 18.23 ± 1.06 kg of body weight were administered an oral gavage of water (control) or a 3 mmol/kg of Glu, Ile, Leu, Lys, Phe, or glucose (positive control for GLP-1 release) following an overnight fasting during 5 consecutive days using an incomplete latin square design. Blood samples were collected from the jugular vein before (-5 min, baseline value) and after the gavage (5, 15, 30, 60 and 90 min) to assess CCK and GLP-1 plasma levels. Pigs administered the oral gavage of Leu (P < 0.05), or Lys (P < 0.1) had increased levels of plasma CCK from 0 to 90 min post-gavage when compared to the control. A strong association (P < 0.001) was observed between GLP-1 plasma levels with Phe intake. The impact was significant starting 30 min post-gavage and was sustained until the end of the experiment (90 min post-gavage). Glucose administration increased GLP-1 early after the intake at the 5 min mark (P < 0.1). A positive correlation (P < 0.05, r = 0.89) driven by the impact of Phe at the 60 to 90 min post-gavage was identified between CCK and GLP-1 indicating feedback mechanisms between proximal and distal small intestine. In conclusion, oral gavages of Leu and Lys increased anorexigenic hormone CCK plasma levels in pigs. Phe caused a significant long-lasting increase in incretin GLP-1 plasma levels. Blood CCK and GLP-1 concentrations in Phe gavaged pigs were positively correlated indicating a potential feedback mechanism between proximal (CCK) and distal (GLP-1) small intestine. The present results are compatible with the known anorexigenic effects of excess dietary Leu and Lys, and the insulinotropic effect of Phe in pigs. These results demonstrate the relevance of accurate feed formulation practices particularly in post weaning pigs.
Keywords: amino acid; blood; cholecystokinin; glucagon-like peptide 1; oral gavage; pig.
Plain language summary
Previous ex vivo studies showed how the amino acids (AA) Lys, Leu, Ile, Phe, and Glu increased satiety peptide cholecystokinin (CCK) and/or insulinotropic hormone glucagon-like peptide 1 (GLP-1) model in pigs. The objective of this study was to validate the ex vivo model by testing the AA of interest in live pigs. Following the oral administration by gavage Leu increased plasma CCK compared to water. Phe showed a sustained long-lasting increase in GLP-1 plasma levels appearing 30 min after the gavage. A positive correlation between CCK and GLP-1 blood levels was observed for Phe treated pigs between 60 and 90 min after the treatment indicating that GLP-1 may induce the release of CCK in the small intestine via feedback mechanisms. The results also showed a trend for Lys increasing CCK congruent with previous data reporting an inhibition of appetite by dietary excess of this AA. These findings are relevant for commercial feeding practices since Lys is often supplemented and dietary Leu is commonly high in pig feeds. Finally, our results highlight the relevance of aromatic AA (i.e., Phe), in pig nutrition that deserves additional attention. There is significant room for improving the understanding of optimal AA levels in pig feeds.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Society of Animal Science.
Conflict of interest statement
The authors declare there is no conflict of interest.
Figures

Similar articles
-
Excess dietary Lys reduces feed intake, stimulates jejunal CCK secretion and alters essential and non-essential blood AA profile in pigs.J Anim Sci Biotechnol. 2024 Feb 18;15(1):24. doi: 10.1186/s40104-023-00971-9. J Anim Sci Biotechnol. 2024. PMID: 38369505 Free PMC article.
-
An oral gavage of lysine elicited early satiation while gavages of lysine, leucine, or isoleucine prolonged satiety in pigs.J Anim Sci. 2022 Dec 1;100(12):skac361. doi: 10.1093/jas/skac361. J Anim Sci. 2022. PMID: 36315475 Free PMC article.
-
CCK and GLP-1 release in response to proteinogenic amino acids using a small intestine ex vivo model in pigs.J Anim Sci. 2022 Apr 1;100(4):skac093. doi: 10.1093/jas/skac093. J Anim Sci. 2022. PMID: 35323927 Free PMC article.
-
EndoBarrier gastrointestinal liner. Delineation of underlying mechanisms and clinical effects.Dan Med J. 2016 Nov;63(11):B5309. Dan Med J. 2016. PMID: 27808040 Review.
-
Incretin physiology beyond glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: cholecystokinin and gastrin peptides.Acta Physiol (Oxf). 2011 Apr;201(4):405-11. doi: 10.1111/j.1748-1716.2010.02235.x. Acta Physiol (Oxf). 2011. PMID: 21392266 Review.
Cited by
-
Excess dietary Lys reduces feed intake, stimulates jejunal CCK secretion and alters essential and non-essential blood AA profile in pigs.J Anim Sci Biotechnol. 2024 Feb 18;15(1):24. doi: 10.1186/s40104-023-00971-9. J Anim Sci Biotechnol. 2024. PMID: 38369505 Free PMC article.
-
Examining the Direct and Indirect Effects of Postprandial Amino Acid Responses on Markers of Satiety following the Acute Consumption of Lean Beef-Rich Meals in Healthy Women with Overweight.Nutrients. 2024 May 31;16(11):1718. doi: 10.3390/nu16111718. Nutrients. 2024. PMID: 38892651 Free PMC article.
References
-
- Da Silva, E. C., De Jager N., Burgos W., Reverter A., Perez-Enciso M., and Roura E.. . 2014. Characterization of the Porcine GPCR Nutrient Sensor and Taste Receptor Gene Repertoire across international and local domestic breeds and wild populations. BMC Genom. 15:1057. doi:10.1186/1471-2164-15-1057 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

