Maternal Mineral Nutrition Regulates Fetal Genomic Programming in Cattle: A Review
- PMID: 37233634
- PMCID: PMC10223334
- DOI: 10.3390/metabo13050593
Maternal Mineral Nutrition Regulates Fetal Genomic Programming in Cattle: A Review
Abstract
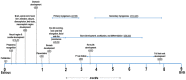
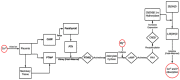
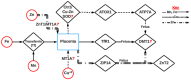
Maternal mineral nutrition during the critical phases of fetal development may leave lifetime impacts on the productivity of an individual. Most research within the developmental origins of the health and disease (DOHaD) field is focused on the role of macronutrients in the genome function and programming of the developing fetus. On the other hand, there is a paucity of knowledge about the role of micronutrients and, specifically, minerals in regulating the epigenome of livestock species, especially cattle. Therefore, this review will address the effects of the maternal dietary mineral supply on the fetal developmental programming from the embryonic to the postnatal phases in cattle. To this end, we will draw a parallel between findings from our cattle model research with data from model animals, cell lines, and other livestock species. The coordinated role and function of different mineral elements in feto-maternal genomic regulation underlies the establishment of pregnancy and organogenesis and, ultimately, affects the development and functioning of metabolically important tissues, such as the fetal liver, skeletal muscle, and, importantly, the placenta. Through this review, we will delineate the key regulatory pathways involved in fetal programming based on the dietary maternal mineral supply and its crosstalk with epigenomic regulation in cattle.
Keywords: developmental biology; epigenetics; epigenome; essential nutrients; genetics; macrominerals; microminerals; restricted nutrition.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


References
-
- Minelli A. Animal Development, an Open-Ended Segment of Life. Biol. Theory. 2011;6:4–15. doi: 10.1007/s13752-011-0002-6. - DOI
-
- Love A.C. Explaining the Ontogeny of Form: Philosophical Issue. Wiley Online Library; Hoboken, NJ, USA: 2008.
Publication types
LinkOut - more resources
Full Text Sources

