Glucolipotoxic Stress-Induced Mig6 Desensitizes EGFR Signaling and Promotes Pancreatic Beta Cell Death
- PMID: 37233668
- PMCID: PMC10222246
- DOI: 10.3390/metabo13050627
Glucolipotoxic Stress-Induced Mig6 Desensitizes EGFR Signaling and Promotes Pancreatic Beta Cell Death
Abstract
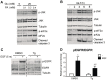
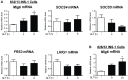
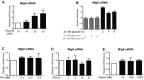
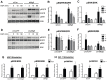
A loss of functional beta cell mass is a final etiological event in the development of frank type 2 diabetes (T2D). To preserve or expand beta cells and therefore treat/prevent T2D, growth factors have been considered therapeutically but have largely failed to achieve robust clinical success. The molecular mechanisms preventing the activation of mitogenic signaling pathways from maintaining functional beta cell mass during the development of T2D remain unknown. We speculated that endogenous negative effectors of mitogenic signaling cascades impede beta cell survival/expansion. Thus, we tested the hypothesis that a stress-inducible epidermal growth factor receptor (EGFR) inhibitor, mitogen-inducible gene 6 (Mig6), regulates beta cell fate in a T2D milieu. To this end, we determined that: (1) glucolipotoxicity (GLT) induces Mig6, thereby blunting EGFR signaling cascades, and (2) Mig6 mediates molecular events regulating beta cell survival/death. We discovered that GLT impairs EGFR activation, and Mig6 is elevated in human islets from T2D donors as well as GLT-treated rodent islets and 832/13 INS-1 beta cells. Mig6 is essential for GLT-induced EGFR desensitization, as Mig6 suppression rescued the GLT-impaired EGFR and ERK1/2 activation. Further, Mig6 mediated EGFR but not insulin-like growth factor-1 receptor nor hepatocyte growth factor receptor activity in beta cells. Finally, we identified that elevated Mig6 augmented beta cell apoptosis, as Mig6 suppression reduced apoptosis during GLT. In conclusion, we established that T2D and GLT induce Mig6 in beta cells; the elevated Mig6 desensitizes EGFR signaling and induces beta cell death, suggesting Mig6 could be a novel therapeutic target for T2D.
Keywords: Errfi1; diabetes; islet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hakonen E., Ustinov J., Mathijs I., Palgi J., Bouwens L., Miettinen P.J., Otonkoski T. Epidermal growth factor (EGF)-receptor signalling is needed for murine beta cell mass expansion in response to high-fat diet and pregnancy but not after pancreatic duct ligation. Diabetologia. 2011;54:1735–1743. doi: 10.1007/s00125-011-2153-1. - DOI - PubMed
-
- Cousin S.P., Hugl S.R., Wrede C.E., Kajio H., Myers M.G., Jr., Rhodes C.J. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

