Molecular Mechanisms of Western Diet-Induced Obesity and Obesity-Related Carcinogenesis-A Narrative Review
- PMID: 37233716
- PMCID: PMC10222258
- DOI: 10.3390/metabo13050675
Molecular Mechanisms of Western Diet-Induced Obesity and Obesity-Related Carcinogenesis-A Narrative Review
Abstract
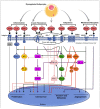
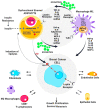
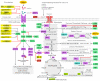
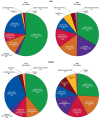
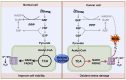
The present study aims to provide a narrative review of the molecular mechanisms of Western diet-induced obesity and obesity-related carcinogenesis. A literature search of the Cochrane Library, Embase and Pubmed databases, Google Scholar and the grey literature was conducted. Most of the molecular mechanisms that induce obesity are also involved in the twelve Hallmarks of Cancer, with the fundamental process being the consumption of a highly processed, energy-dense diet and the deposition of fat in white adipose tissue and the liver. The generation of crown-like structures, with macrophages surrounding senescent or necrotic adipocytes or hepatocytes, leads to a perpetual state of chronic inflammation, oxidative stress, hyperinsulinaemia, aromatase activity, activation of oncogenic pathways and loss of normal homeostasis. Metabolic reprogramming, epithelial mesenchymal transition, HIF-1α signalling, angiogenesis and loss of normal host immune-surveillance are particularly important. Obesity-associated carcinogenesis is closely related to metabolic syndrome, hypoxia, visceral adipose tissue dysfunction, oestrogen synthesis and detrimental cytokine, adipokine and exosomal miRNA release. This is particularly important in the pathogenesis of oestrogen-sensitive cancers, including breast, endometrial, ovarian and thyroid cancer, but also 'non-hormonal' obesity-associated cancers such as cardio-oesophageal, colorectal, renal, pancreatic, gallbladder and hepatocellular adenocarcinoma. Effective weight loss interventions may improve the future incidence of overall and obesity-associated cancer.
Keywords: GLP-1; HIF-1α; NASH; bariatric surgery; breast cancer; colorectal carcinoma; crown-like structure (CLS); cytokine; exosome; hypoxia; leptin; macrophage polarization; metabolic syndrome; obesity; senescence.
Conflict of interest statement
R.B.W. has received funding for research, education and attendance at scientific meetings from MSD, Fisher and Paykel, Ethicon and Medtronic.
Figures





References
-
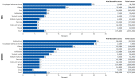
- Australian Institute of Health and Welfare Cancer Data in Australia. Cat. No. CAN 122. [(accessed on 29 November 2022)]; Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/data.
-
- Murray C.J., Aravkin A.Y., Zheng P., Abbafati C., Abbas K.M., Abbasi-Kangevari M., Abd-Allah F., Abdelalim A., Abdollahi M., Abdollahpour I. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study. Lancet. 2019;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

