Micropipette aspiration as a tool for single-particle X-ray imaging and diffraction
- PMID: 37233735
- PMCID: PMC10325018
- DOI: 10.1107/S1600577523003685
Micropipette aspiration as a tool for single-particle X-ray imaging and diffraction
Abstract
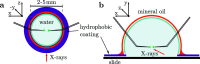
A sample environment and manipulation tool is presented for single-particle X-ray experiments in an aqueous environment. The system is based on a single water droplet, positioned on a substrate that is structured by a hydrophobic and hydrophilic pattern to stabilize the droplet position. The substrate can support several droplets at a time. Evaporation is prevented by covering the droplet by a thin film of mineral oil. In this windowless fluid which minimizes background signal, single particles can be probed and manipulated by micropipettes, which can easily be inserted and steered in the droplet. Holographic X-ray imaging is shown to be well suited to observe and monitor the pipettes, as well as the droplet surface and the particles. Aspiration and force generation are also enabled based on an application of controlled pressure differences. Experimental challenges are addressed and first results are presented, obtained at two different undulator endstations with nano-focused beams. Finally, the sample environment is discussed in view of future coherent imaging and diffraction experiments with synchrotron radiation and single X-ray free-electron laser pulses.
Keywords: holographic X-ray imaging; micropipette aspiration; sample delivery of biophysical samples; single-particle diffraction.
open access.
Figures






Similar articles
-
Macromolecular structures probed by combining single-shot free-electron laser diffraction with synchrotron coherent X-ray imaging.Nat Commun. 2014 May 2;5:3798. doi: 10.1038/ncomms4798. Nat Commun. 2014. PMID: 24786694
-
X-ray laser-induced electron dynamics observed by femtosecond diffraction from nanocrystals of Buckminsterfullerene.Sci Adv. 2016 Sep 9;2(9):e1601186. doi: 10.1126/sciadv.1601186. eCollection 2016 Sep. Sci Adv. 2016. PMID: 27626076 Free PMC article.
-
Digital in-line X-ray holography with zone plates.Ultramicroscopy. 2011 Jul;111(8):1131-6. doi: 10.1016/j.ultramic.2011.02.002. Epub 2011 Feb 12. Ultramicroscopy. 2011. PMID: 21740876
-
X-Ray Free-Electron Lasers for the Structure and Dynamics of Macromolecules.Annu Rev Biochem. 2019 Jun 20;88:35-58. doi: 10.1146/annurev-biochem-013118-110744. Epub 2019 Jan 2. Annu Rev Biochem. 2019. PMID: 30601681 Review.
-
Coherent diffractive imaging of biological samples at synchrotron and free electron laser facilities.J Biotechnol. 2010 Sep 15;149(4):229-37. doi: 10.1016/j.jbiotec.2010.01.024. Epub 2010 Feb 10. J Biotechnol. 2010. PMID: 20149827 Review.
References
-
- Ashkin, A. (1970). Phys. Rev. Lett. 24, 156–159.
-
- Ashkin, A. (1978). Phys. Rev. Lett. 40, 729–732.
-
- Ashkin, A. & Dziedzic, J. (1987). Science, 235, 1517–1520. - PubMed
-
- Beerlink, A., Mell, M., Tolkiehn, M. & Salditt, T. (2009). Appl. Phys. Lett. 95, 203703.
-
- Beerlink, A., Thutupalli, S., Mell, M., Bartels, M., Cloetens, P., Herminghaus, S. & Salditt, T. (2012). Soft Matter, 8, 4595–4601.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

