Efficacy and Safety of Apremilast for the Treatment of Japanese Patients with Palmoplantar Pustulosis: Results from a Phase 2, Randomized, Placebo-Controlled Study
- PMID: 37233897
- PMCID: PMC10213585
- DOI: 10.1007/s40257-023-00788-2
Efficacy and Safety of Apremilast for the Treatment of Japanese Patients with Palmoplantar Pustulosis: Results from a Phase 2, Randomized, Placebo-Controlled Study
Erratum in
-
Author Correction: Efficacy and Safety of Apremilast for the Treatment of Japanese Patients with Palmoplantar Pustulosis: Results from a Phase 2, Randomized, Placebo-Controlled Study.Am J Clin Dermatol. 2024 Jan;25(1):165-167. doi: 10.1007/s40257-023-00825-0. Am J Clin Dermatol. 2024. PMID: 38060175 Free PMC article. No abstract available.
Abstract
Background: Palmoplantar pustulosis (PPP) is a pruritic, painful, recurrent, and chronic dermatitis with limited therapeutic options.
Objective: To evaluate the efficacy and safety of apremilast for the treatment of Japanese patients with PPP and inadequate response to topical treatment.
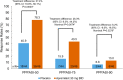
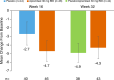
Methods: This phase 2, randomized, double-blind, placebo-controlled study enrolled patients with Palmoplantar Pustulosis Area and Severity Index (PPPASI) total score ≥ 12 and moderate or severe pustules/vesicles on the palm or sole (PPPASI pustule/vesicle severity score ≥ 2) at screening and baseline with an inadequate response to topical treatment. Patients were randomized (1:1) to apremilast 30 mg twice daily or placebo for 16 weeks, followed by a 16-week extension phase during which all patients received apremilast. The primary endpoint was achievement of PPPASI-50 response (≥ 50% improvement from baseline in PPPASI). Key secondary endpoints included change from baseline in PPPASI total score, Palmoplantar Pustulosis Severity Index (PPSI), and patient's visual analog scale (VAS) for PPP symptoms (pruritus and discomfort/pain).
Results: A total of 90 patients were randomized (apremilast: 46; placebo: 44). A significantly greater proportion of patients achieved PPPASI-50 at week 16 with apremilast versus placebo (P = 0.0003). Patients receiving apremilast showed greater improvement in PPPASI at week 16 versus placebo (nominal P = 0.0013), as well as PPSI and patient-reported pruritus and discomfort/pain (nominal P ≤ 0.001 for all). Improvements were sustained through week 32 with apremilast treatment. The most common treatment-emergent adverse events included diarrhea, abdominal discomfort, headache, and nausea.
Conclusions: Apremilast treatment demonstrated greater improvements in disease severity and patient-reported symptoms versus placebo at week 16 in Japanese patients with PPP with sustained improvements through week 32. No new safety signals were observed.
Clinicaltrials: GOV: NCT04057937.
© 2023. The Author(s).
Conflict of interest statement
Tadashi Terui has received research grants, consulting fees, and/or speaker’s fees from AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Eisai, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis, and Taiho Pharmaceutical. Yukari Okubo has received grants or contracts from AbbVie, Eisai, Jimro, Maruho, Shiseido, Sun Pharma, and Torii; consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, Kyowa Kirin, LEO Pharma, Maruho, Pfizer, Sun Pharma, and UCB Pharma; and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, JIMRO, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Pfizer, Sanofi, Sun Pharma, Taiho, Tanabe-Mitsubishi, Torii and UCB Pharma. Satomi Kobayashi has received research grants from Kyowa Kirin; received honoraria from AbbVie, Eli Lilly, Janssen Pharma, Maruho Pharmaceutical, Novartis, and Taiho Pharmaceutical; and participated in clinical trials for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Pharma, Kyowa Kirin, Maruho Pharmaceutical, and Pfizer. Shigetoshi Sano has received research grants from Kaken, Maruho, Nihon, Nippon Zoki, Sanofi, Taiho, and Torii; and honoraria from AbbVie, Eisai, Eli Lilly, Janssen Pharma, Kyowa Kirin, Maruho, Sun Pharma, Taiho, and UCB. Akimichi Morita has received research grants, consulting fees, and/or speaker’s fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi-Iko, Nippon Kayaku, Novartis, Pfizer, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, Ushio, and UCB Pharma. Shinichi Imafuku has received grants and/or personal fees from AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly, GSK, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Novartis, Sun Pharma, Taiho Pharmaceutical, Tanabe-Mitsubishi, Torii Pharmaceutical, and UCB Japan. Yayoi Tada has received honoraria and/or grants from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb KK, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Novartis Pharma, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Pharma. Masatoshi Abe has received research grants, consulting fees, speaker fees, and/or participated in clinical trials for Celgene and Maruho. Masafumi Yaguchi, Natsuka Uehara, Takahiro Handa, and Masayuki Tanaka are employees of Amgen K.K. Wendy Zhang and Maria Paris are employees and stockholders of Amgen Inc. Masamoto Murakami has received research grants from AbbVie, ARISTEA Therapeutics, Eisai, Eli Lilly, Kyowa Kirin, and Novartis Pharma; honoraria from AbbVie, Amgen Inc., Boehringer Ingelheim, Celgene, Eisai, Eli Lilly, Janssen Pharma, Kyowa Kirin, Maruho, Novartis Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical; and participated in clinical trials for AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen Pharma, Maruho, and Novartis Pharma.
Figures


References
-
- Liang Y, Xing X, Beamer MA, Swindell WR, Sarkar MK, Roberts LW, et al. Six-transmembrane epithelial antigens of the prostate comprise a novel inflammatory nexus in patients with pustular skin disorders. J Allergy Clin Immunol. 2017;139(4):1217–1227. doi: 10.1016/j.jaci.2016.10.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

