Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma
- PMID: 37234171
- PMCID: PMC10206168
- DOI: 10.3389/fimmu.2023.1166497
Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma
Abstract
Introduction: Cholangiocarcinoma (CCA) is a malignant tumor of the biliary epithelium with a poor prognosis. The lack of biomarkers to predict therapeutic response and prognosis is one of the major challenges for CCA treatment. Tertiary lymphoid structures (TLS) provide a local and pivotal microenvironment for tumor immune responses. The prognostic value and clinical relevance of TLS in CCA remain unclear. We aimed to explore the characteristics and clinical significance of TLS in CCA.
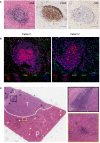
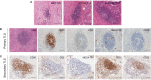
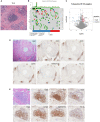
Methods: We investigated the prognostic value and clinical relevance of TLS in CCA using a surgery cohort containing 471 CCA patients (cohort 1) and an immunotherapy cohort containing 100 CCA patients (cohort 2). Hematoxylin and eosin (H&E) and immunohistochemical (IHC) staining were used to evaluate the maturity of TLS. Multiplex IHC (mIHC) was employed to characterize the composition of TLS.
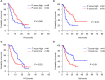
Results: Different maturity of TLS were observed in CCA tissue sections. Strong staining of the four-gene signature including PAX5, TCL1A, TNFRSF13C, and CD79A were found in TLS regions. A high density of intra-tumoral TLS (T-score high) were significantly correlated with longer overall survival (OS) both in CCA cohort 1 (p = 0.002) and cohort 2 (p = 0.01), whereas a high density of peri-tumoral TLS (P-score high) were associated with shorter OS in these two cohorts (p = 0.003 and p = 0.03, respectively).
Conclusion: The established four-gene signature efficiently identified the TLS in CCA tissues. The abundance and spatial distribution of TLS were significantly correlated with the prognosis and immune checkpoint inhibitors (ICIs) immunotherapy response of CCA patients. The presence of intra-tumoral TLS are positive prognostic factors for CCA, which provide a theoretical basis for the future diagnosis and treatment of CCA.
Keywords: cholangiocarcinoma; immune checkpoint inhibitors; prognosis; tertiary lymphoid structures; tumor microenvironment.
Copyright © 2023 Shang, Jiang, Lu, Wang, Cui, Pan, Xu, Pei, Ding, Feng, Lin, Li, Tan, Feng, Dong, Wang and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. . Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology (2020) 72(3):965–81. doi: 10.1002/hep.31092 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

