pH-sensing hybrid hydrogels for non-invasive metabolism monitoring in tumor spheroids
- PMID: 37234366
- PMCID: PMC10205545
- DOI: 10.1016/j.mtbio.2023.100655
pH-sensing hybrid hydrogels for non-invasive metabolism monitoring in tumor spheroids
Abstract
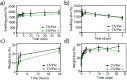
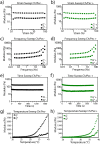
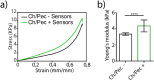
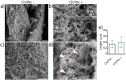
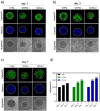
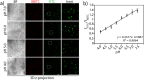
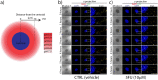
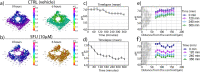
The constant increase in cancer incidence and mortality pushes biomedical research towards the development of in vitro 3D systems able to faithfully reproduce and effectively probe the tumor microenvironment. Cancer cells interact with this complex and dynamic architecture, leading to peculiar tumor-associated phenomena, such as acidic pH conditions, rigid extracellular matrix, altered vasculature, hypoxic condition. Acidification of extracellular pH, in particular, is a well-known feature of solid tumors, correlated to cancer initiation, progression, and resistance to therapies. Monitoring local pH variations, non-invasively, during cancer growth and in response to drug treatment becomes extremely important for understanding cancer mechanisms. Here, we describe a simple and reliable pH-sensing hybrid system, based on a thermoresponsive hydrogel embedding optical pH sensors, that we specifically apply for non-invasive and accurate metabolism monitoring in colorectal cancer (CRC) spheroids. First, the physico-chemical properties of the hybrid sensing platform, in terms of stability, rheological and mechanical properties, morphology and pH sensitivity, were fully characterized. Then, the proton gradient distribution in the spheroids proximity, in the presence or absence of drug treatment, was quantified over time by time lapse confocal light scanning microscopy and automated segmentation pipeline, highlighting the effects of the drug treatment in the extracellular pH. In particular, in the treated CRC spheroids the acidification of the microenvironment resulted faster and more pronounced over time. Moreover, a pH gradient distribution was detected in the untreated spheroids, with more acidic values in proximity of the spheroids, resembling the cell metabolic features observed in vivo in the tumor microenvironment. These findings promise to shed light on mechanisms of regulation of proton exchanges by cellular metabolism being essential for the study of solid tumors in 3D in vitro models and the development of personalized medicine approaches.
Keywords: Automated computational analysis; Image segmentation; Ratiometric pH sensing; Silica microparticles; Thermoresponsive hydrogels; Tumor models.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Castro F., Leite Pereira C., Helena Macedo M., Almeida A., José Silveira M., Dias S., Patrícia Cardoso A., José Oliveira M., Sarmento B. Advances on colorectal cancer 3D models: the needed translational technology for nanomedicine screening. Adv. Drug Deliv. Rev. 2021;175 doi: 10.1016/j.addr.2021.06.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources

