Dry loop-mediated isothermal amplification assay for detection of SARS-CoV-2 from clinical specimens
- PMID: 37234399
- PMCID: PMC10206895
- DOI: 10.20407/fmj.2022-003
Dry loop-mediated isothermal amplification assay for detection of SARS-CoV-2 from clinical specimens
Abstract
Objectives: To establish a point-of-care test for coronavirus disease 2019 (COVID-19), we developed a dry loop-mediated isothermal amplification (LAMP) method to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA.
Methods: We carried out reverse transcription (RT)-LAMP using the Loopamp SARS-CoV-2 Detection kit (Eiken Chemical, Tokyo, Japan). The entire mixture, except for the primers, is dried and immobilized inside the tube lid.
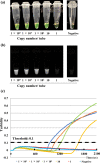
Results: To determine the specificity of the kit, 22 viruses associated with respiratory infections, including SARS-CoV-2, were tested. The sensitivity of this assay, determined by either a real-time turbidity assay or colorimetric change of the reaction mixture, as evaluated by the naked eye or under illumination with ultraviolet light, was 10 copies/reaction. No LAMP product was detected in reactions performed with RNA from any pathogens other than SARS-CoV-2. After completing an initial validation analysis, we analyzed 24 nasopharyngeal swab specimens collected from patients suspected to have COVID-19. Of the 24 samples, 19 (79.2%) were determined by real-time RT-PCR analysis as being positive for SARS-CoV-2 RNA. Using the Loopamp SARS-CoV-2 Detection kit, we detected SARS-CoV-2 RNA in 15 (62.5%) of the 24 samples. Thus, the sensitivity, specificity, positive predictive value, and negative predictive values of the Loopamp 2019-CoV-2 detection reagent kit were 78.9%, 100%, 100%, and 55.6%, respectively.
Conclusions: The dry LAMP method for detecting SARS-CoV-2 RNA is fast and easy to use, and its reagents can be stored at 4°C, solving the cold chain problem; thus, it represents a promising tool for COVID-19 diagnosis in developing countries.
Keywords: COVID-19; LAMP; Real-time RT-PCR; SARS-CoV-2.
Conflict of interest statement
All authors declare no conflict of interest with regard to this work.
Figures
Similar articles
-
Evaluation of rapid diagnosis of novel coronavirus disease (COVID-19) using loop-mediated isothermal amplification.J Clin Virol. 2020 Aug;129:104446. doi: 10.1016/j.jcv.2020.104446. Epub 2020 May 21. J Clin Virol. 2020. PMID: 32512376 Free PMC article.
-
Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay.Clin Microbiol Infect. 2020 Jun;26(6):773-779. doi: 10.1016/j.cmi.2020.04.001. Epub 2020 Apr 8. Clin Microbiol Infect. 2020. PMID: 32276116 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
Detection of SARS-CoV-2 and the L452R spike mutation using reverse transcription loop-mediated isothermal amplification plus bioluminescent assay in real-time (RT-LAMP-BART).PLoS One. 2022 Mar 21;17(3):e0265748. doi: 10.1371/journal.pone.0265748. eCollection 2022. PLoS One. 2022. PMID: 35312732 Free PMC article.
-
The Use of Loop-Mediated Isothermal Amplification (TB-LAMP) for the Diagnosis of Pulmonary Tuberculosis: Policy Guidance.Geneva: World Health Organization; 2016. Geneva: World Health Organization; 2016. PMID: 27606385 Free Books & Documents. Review.
Cited by
-
Simple and Rapid Colorimetric Detection of Canine Parainfluenza Virus 5 (Orthorubulavirus mammalis) Using a Reverse-Transcription Loop-Mediated Isothermal Amplification Assay.Pathogens. 2023 Jul 8;12(7):921. doi: 10.3390/pathogens12070921. Pathogens. 2023. PMID: 37513767 Free PMC article.
-
Clinical Performance of the Reverse Transcription-Loop-Mediated Isothermal Amplification Assay for the Diagnosis of COVID-19 in a Thai Community Hospital at the Thailand-Myanmar Border.Cureus. 2024 Feb 19;16(2):e54447. doi: 10.7759/cureus.54447. eCollection 2024 Feb. Cureus. 2024. PMID: 38510857 Free PMC article.
-
Diagnostic accuracy of LAMP versus PCR over the course of SARS-CoV-2 infection.Int J Infect Dis. 2021 Jun;107:195-200. doi: 10.1016/j.ijid.2021.04.018. Epub 2021 Apr 20. Int J Infect Dis. 2021. PMID: 33862213 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
References
LinkOut - more resources
Full Text Sources
Miscellaneous

