Role of serotype and virulence determinants of Streptococcus pyogenes biofilm bacteria in internalization and persistence in epithelial cells in vitro
- PMID: 37234777
- PMCID: PMC10206268
- DOI: 10.3389/fcimb.2023.1146431
Role of serotype and virulence determinants of Streptococcus pyogenes biofilm bacteria in internalization and persistence in epithelial cells in vitro
Abstract
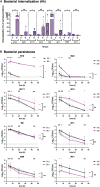
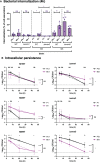
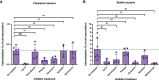
Streptococcus pyogenes causes a multitude of local and systemic infections, the most common being pharyngitis in children. Recurrent pharyngeal infections are common and are thought to be due to the re-emergence of intracellular GAS upon completion of antibiotic treatment. The role of colonizing biofilm bacteria in this process is not fully clear. Here, live respiratory epithelial cells were inoculated with broth-grown or biofilm bacteria of different M-types, as well as with isogenic mutants lacking common virulence factors. All M-types tested adhered to and were internalized into epithelial cells. Interestingly, internalization and persistence of planktonic bacteria varied significantly between strains, whereas biofilm bacteria were internalized in similar and higher numbers, and all strains persisted beyond 44 hours, showing a more homogenous phenotype. The M3 protein, but not the M1 or M5 proteins, was required for optimal uptake and persistence of both planktonic and biofilm bacteria inside cells. Moreover, the high expression of capsule and SLO inhibited cellular uptake and capsule expression was required for intracellular survival. Streptolysin S was required for optimal uptake and persistence of M3 planktonic bacteria, whereas SpeB improved intracellular survival of biofilm bacteria. Microscopy of internalized bacteria showed that planktonic bacteria were internalized in lower numbers as individual or small clumps of bacteria in the cytoplasm, whereas GAS biofilm bacteria displayed a pattern of perinuclear localization of bacterial aggregates that affected actin structure. Using inhibitors targeting cellular uptake pathways, we confirmed that planktonic GAS mainly uses a clathrin-mediated uptake pathway that also required actin and dynamin. Clathrin was not involved in biofilm internalization, but internalization required actin rearrangement and PI3 kinase activity, possibly suggesting macropinocytosis. Together these results provide a better understanding of the potential mechanisms of uptake and survival of various phenotypes of GAS bacteria relevant for colonization and recurrent infection.
Keywords: Streptococcus pyogenes; adherence; epithelial cells; internalization; localization; persistence; uptake pathways; virulence factors.
Copyright © 2023 Alamiri, André, De, Nordenfelt and Hakansson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ashbaugh C. D., Moser T. J., Shearer M. H., White G. L., Kennedy R. C., Wessels M. R. (2000). Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group a streptococcal pharyngeal infection. Cell Microbiol. 2, 283–292. doi: 10.1046/j.1462-5822.2000.00050.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

