Genetic Variants Associated with Poor Responsiveness to Sulfonylureas in Filipinos with Type 2 Diabetes Mellitus
- PMID: 37234931
- PMCID: PMC10207869
- DOI: 10.15605/jafes.037.S8
Genetic Variants Associated with Poor Responsiveness to Sulfonylureas in Filipinos with Type 2 Diabetes Mellitus
Abstract
Introduction: Sulfonylureas (SUs) are commonly used drugs for type 2 diabetes mellitus (T2DM) in the Philippines. This study aimed to associate genetic variants with poor response to gliclazide and glimepiride among Filipinos.
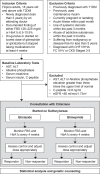
Methodology: Two independent, dichotomous longitudinal substudies enrolled 139 and 113 participants in the gliclazide and glimepiride substudies, respectively. DNA from blood samples underwent customized genotyping for candidate genes using microarray. Allelic and genotypic features and clinical associations were determined using exact statistical methods.
Results: Three months after sulfonylurea monotherapy, 18 (13%) were found to be poorly responsive to gliclazide, while 7 (6%) had poor response to glimepiride. Seven genetic variants were nominally associated (p<0.05) with poor gliclazide response, while three variants were nominally associated with poor glimepiride response. For gliclazide response, 3 carboxypeptidase-associated variants (rs319952 and rs393994 of AGBL4 and rs2229437 of PRCP) had the highest genotypic association; other variants include rs9806699, rs7119, rs6465084 and rs1234315. For glimepiride response, 2 variants were nominally associated: CLCN6-NPPA-MTHFR gene cluster - rs5063 and rs17367504 - and rs2299267 from the PON2 loci.
Conclusion: Genetic variants were found to have a nominal association with sulfonylurea response among Filipinos. These findings can guide for future study directions on pharmacotherapeutic applications for sulfonylurea treatment in this population.
Keywords: Filipino; genetic variants; gliclazide; glimepiride; resistance; sulfonylureas.
© 2022 Journal of the ASEAN Federation of Endocrine Societies.
Figures


References
-
- Jimeno C, Sobrepeña L, Mirasol R. DiabCare 2008: Survey on glycaemic control and the status of diabetes care and complications among patients with type 2 diabetes mellitus in the Philippines. Philipp J Intern Med. 2012;50(1):15-22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
