Marketing authorisation and pricing of FDA-approved cancer drugs in Brazil: a retrospective analysis
- PMID: 37235087
- PMCID: PMC10206192
- DOI: 10.1016/j.lana.2023.100506
Marketing authorisation and pricing of FDA-approved cancer drugs in Brazil: a retrospective analysis
Abstract
Background: Most cancer drugs enter the US market first. US Food and Drug Administration (FDA) approvals of new cancer drugs may influence regulatory decisions in other settings. The study examined whether characteristics of available evidence at FDA approval influenced time-to-marketing authorisation (MA) in Brazil, and price differences between the two countries.
Methods: All new FDA-approved cancer drugs from 2010 to 2019 were matched to drugs with MA and prices approved in Brazil by December 2020. Characteristics of main studies, availability of randomised controlled trials (RCTs), overall survival (OS) benefit, added therapeutic benefit, and prices were compared.
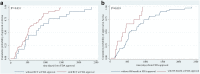
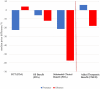
Findings: Fifty-six FDA-approved cancer drugs with matching indications received a MA at the Brazilian Health Regulatory Agency (Anvisa) after a median of 522 days following US approval (IQR: 351-932). Earlier authorisation in Brazil was associated with availability of RCT (median: 506 vs 760 days, p = 0.031) and evidence of OS benefit (390 vs 543 days, p = 0.019) at FDA approval. At Brazilian marketing authorisation, a greater proportion of cancer drugs had main RCTs (75% vs 60.7%) and OS benefit (42.9% vs 21.4%) than that in the US. Twenty-eight (50%) drugs did not demonstrate added therapeutic benefit over drugs for the same indication in Brazil. Median approved prices of new cancer drugs were 12.9% lower in Brazil compared to the US (adjusted by Purchasing Power Parity). However, for drugs with added therapeutic benefit median prices were 5.9% higher in Brazil compared to the US, while 17.9% lower for those without added benefit.
Interpretation: High-quality clinical evidence accelerated the availability of cancer medicines in Brazil. The combination of marketing and pricing authorisation in Brazil may favour the approval of cancer drugs with better supporting evidence, and more meaningful clinical benefit albeit with variable degree of success in achieving lower prices compared to the US.
Funding: None.
Keywords: Brazil; Cancer; Drug legislation; Health technology assessment; Pharmaceutical preparations; United States.
© 2023 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding this article. HN reports grants from the Health Foundation, the National Institute for Health and Care Research, and UK Research and Innovation outside the submitted work, and consulting fees from the World Health Organization and Pharmaceutical Group of the European Union; HN also reports being an adviser to the Analysis section of The BMJ.
Figures
Similar articles
-
Clinical benefit, reimbursement outcomes, and prices of FDA-approved cancer drugs reviewed through Project Orbis in the USA, Canada, England, and Scotland: a retrospective, comparative analysis.Lancet Oncol. 2024 Aug;25(8):979-988. doi: 10.1016/S1470-2045(24)00286-9. Epub 2024 Jul 11. Lancet Oncol. 2024. PMID: 39004098
-
Approval of Cancer Drugs With Uncertain Therapeutic Value: A Comparison of Regulatory Decisions in Europe and the United States.Milbank Q. 2020 Dec;98(4):1219-1256. doi: 10.1111/1468-0009.12476. Epub 2020 Oct 6. Milbank Q. 2020. PMID: 33021339 Free PMC article.
-
Overall survival benefits of cancer drugs initially approved by the US Food and Drug Administration on the basis of immature survival data: a retrospective analysis.Lancet Oncol. 2024 Jun;25(6):760-769. doi: 10.1016/S1470-2045(24)00152-9. Epub 2024 May 13. Lancet Oncol. 2024. PMID: 38754451
-
Withdrawn accelerated approvals for cancer indications in the USA: what is the marketing authorisation status in the EU?Lancet Oncol. 2023 Sep;24(9):e385-e394. doi: 10.1016/S1470-2045(23)00357-1. Lancet Oncol. 2023. PMID: 37657479 Review.
-
[Cancer: Is it really so different? Particularities of oncologic drugs from the perspective of the pharmaceutical regulatory agency].Z Evid Fortbild Qual Gesundhwes. 2013;107(2):120-8. doi: 10.1016/j.zefq.2013.02.003. Epub 2013 Apr 4. Z Evid Fortbild Qual Gesundhwes. 2013. PMID: 23663906 Review. German.
Cited by
-
Regulatory histories of recently withdrawn ovarian cancer treatment indications of 3 PARP inhibitors in the US and Europe: lessons for the accelerated approval pathway.J Pharm Policy Pract. 2024 Jun 4;17(1):2351003. doi: 10.1080/20523211.2024.2351003. eCollection 2024. J Pharm Policy Pract. 2024. PMID: 38841118 Free PMC article.
-
International Cost-Effectiveness Analysis of Durvalumab in Stage III Non-Small Cell Lung Cancer.JAMA Netw Open. 2024 May 1;7(5):e2413938. doi: 10.1001/jamanetworkopen.2024.13938. JAMA Netw Open. 2024. PMID: 38814640 Free PMC article.
-
Cancer drug indication approvals in China and the United States: a comparison of approval times and clinical benefit, 2001-2020.Lancet Reg Health West Pac. 2024 Apr 2;45:101055. doi: 10.1016/j.lanwpc.2024.101055. eCollection 2024 Apr. Lancet Reg Health West Pac. 2024. PMID: 38590780 Free PMC article.
-
Impact of Funding Source on Long-Term Outcomes in Prostate Cancer: Analysis of a Large Public Database From Sao Paulo, Brazil.JCO Glob Oncol. 2025 Feb;11:e2400271. doi: 10.1200/GO-24-00271. Epub 2025 Feb 6. JCO Glob Oncol. 2025. PMID: 39913877 Free PMC article.
-
Financial challenges of being on long-term, high-cost medications.Neurooncol Pract. 2024 Dec 3;12(Suppl 1):i49-i58. doi: 10.1093/nop/npae098. eCollection 2025 Feb. Neurooncol Pract. 2024. PMID: 39776525 Free PMC article. Review.
References
-
- IQVIA Institute for Human Data Science . IQVIA; 2022. Global oncology trends 2022; p. 65.
LinkOut - more resources
Full Text Sources

