Priorities and Challenges in Methodology for Human Health Risk Assessment from Combined Exposure to Multiple Chemicals
- PMID: 37235216
- PMCID: PMC10224389
- DOI: 10.3390/toxics11050401
Priorities and Challenges in Methodology for Human Health Risk Assessment from Combined Exposure to Multiple Chemicals
Abstract
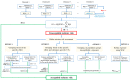
This paper reviews key elements in the assessment of human health effects from combined exposure to multiple chemicals taking into consideration current knowledge and challenges to identify areas where scientific advancement is mostly needed and proposes a decision-making scheme on the basis of existing methods and tools. The assumption of dose addition and estimation of the hazard index (HI) is considered as a starting point in component-based risk assessments. When, based on the generic HI approach, an unacceptable risk is identified, more specific risk assessment options may be implemented sequentially or in parallel depending on problem formulation, characteristics of the chemical group under assessment, exposure levels, data availability and resources. For prospective risk assessments, the reference point index/margin of exposure (RPI/MOET) (Option 1) or modified RPI/normalized MOET (mRPI/nMOET) (Option 2) approaches may be implemented focusing on the specific mixture effect. Relative potency factors (RPFs) may also be used in the RPI approach since a common uncertainty factor for each mixture component is introduced in the assessment. Increased specificity in the risk assessment may also be achieved when exposure of selected population groups is considered (Option 3/exposure). For retrospective risk assessments, human biomonitoring data available for vulnerable population groups (Option 3/susceptibility) may present more focused scenarios for consideration in human health risk management decisions. In data-poor situations, the option of using the mixture assessment factor (MAF) is proposed (Option 4), where an additional uncertainty factor is applied on each mixture component prior to estimating the HI. The magnitude of the MAF may be determined by the number of mixture components, their individual potencies and their proportions in the mixture, as previously reported. It is acknowledged that implementation of currently available methods and tools for human health risk assessment from combined exposure to multiple chemicals by risk assessors will be enhanced by ongoing scientific developments on new approach methodologies (NAMs), integrated approaches to testing and assessment (IATA), uncertainty analysis tools, data sharing platforms, risk assessment software as well as guideline development to meet legislative requirements.
Keywords: chemical mixture; combined exposure; cumulative risk; human risk assessment; risk assessment methodology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- US EPA Supplementary Guidance for Conducting Health Risk Assessment of Mixtures. EPA/630/R-00/002. [(accessed on 19 March 2023)];2000 Available online: https://cfpub.epa.gov/ncea/raf/chem_mix.htm.
-
- EC White Paper—Strategy for a Future Chemicals Policy. [(accessed on 19 March 2023)];COM. 2001 88:1–32. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52001DC008....
-
- WHO Assessment of Combined Exposures to Multiple Chemicals: Report of a WHO/IPCS International Workshop on Aggregate/Cumulative Risk Assessment. IPCS Harmonization Project Document: No. 7. 2009. [(accessed on 18 April 2023)]. Available online: https://apps.who.int/iris/handle/10665/44113.
-
- EC Communication from the Commission to the Council—The combination effects of chemicals, Chemical mixtures. [(accessed on 19 March 2023)];COM. 2012 252:1–10. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52012DC025....
-
- ECHA Guidance on the Information Requirements and Chemical Safety Assessment. Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health. ECHA-2010-G-19-EN. 2012. [(accessed on 19 March 2023)]. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r8....
Publication types
LinkOut - more resources
Full Text Sources

