Microglia mediate neurocognitive deficits by eliminating C1q-tagged synapses in sepsis-associated encephalopathy
- PMID: 37235660
- PMCID: PMC10219600
- DOI: 10.1126/sciadv.abq7806
Microglia mediate neurocognitive deficits by eliminating C1q-tagged synapses in sepsis-associated encephalopathy
Abstract
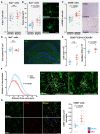
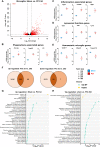
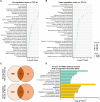
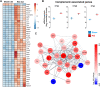
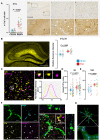
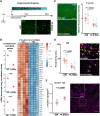
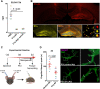
Sepsis-associated encephalopathy (SAE) is a severe and frequent complication of sepsis causing delirium, coma, and long-term cognitive dysfunction. We identified microglia and C1q complement activation in hippocampal autopsy tissue of patients with sepsis and increased C1q-mediated synaptic pruning in a murine polymicrobial sepsis model. Unbiased transcriptomics of hippocampal tissue and isolated microglia derived from septic mice revealed an involvement of the innate immune system, complement activation, and up-regulation of lysosomal pathways during SAE in parallel to neuronal and synaptic damage. Microglial engulfment of C1q-tagged synapses could be prevented by stereotactic intrahippocampal injection of a specific C1q-blocking antibody. Pharmacologically targeting microglia by PLX5622, a CSF1-R inhibitor, reduced C1q levels and the number of C1q-tagged synapses, protected from neuronal damage and synapse loss, and improved neurocognitive outcome. Thus, we identified complement-dependent synaptic pruning by microglia as a crucial pathomechanism for the development of neuronal defects during SAE.
Figures


References
-
- C. Fleischmann, A. Scherag, N. K. J. Adhikari, C. S. Hartog, T. Tsaganos, P. Schlattmann, D. C. Angus, K. Reinhart; International Forum of Acute Care Trialists , Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 193, 259–272 (2016). - PubMed
-
- R. Sonneville, E. de Montmollin, J. Poujade, M. Garrouste-Orgeas, B. Souweine, M. Darmon, E. Mariotte, L. Argaud, F. Barbier, D. Goldgran-Toledano, G. Marcotte, A. S. Dumenil, S. Jamali, G. Lacave, S. Ruckly, B. Mourvillier, J. F. Timsit, Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 43, 1075–1084 (2017). - PubMed
-
- T. E. Gofton, G. B. Young, Sepsis-associated encephalopathy. Nat. Rev. Neurol. 8, 557–566 (2012). - PubMed
-
- A. Semmler, C. N. Widmann, T. Okulla, H. Urbach, M. Kaiser, G. Widman, F. Mormann, J. Weide, K. Fliessbach, A. Hoeft, F. Jessen, C. Putensen, M. T. Heneka, Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry 84, 62–69 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

