Biodegradable Piezoelectric Polymers: Recent Advancements in Materials and Applications
- PMID: 37235849
- PMCID: PMC11469082
- DOI: 10.1002/adhm.202300318
Biodegradable Piezoelectric Polymers: Recent Advancements in Materials and Applications
Abstract
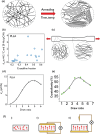
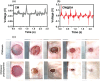
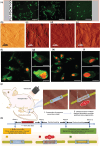
Recent materials, microfabrication, and biotechnology improvements have introduced numerous exciting bioelectronic devices based on piezoelectric materials. There is an intriguing evolution from conventional unrecyclable materials to biodegradable, green, and biocompatible functional materials. As a fundamental electromechanical coupling material in numerous applications, novel piezoelectric materials with a feature of degradability and desired electrical and mechanical properties are being developed for future wearable and implantable bioelectronics. These bioelectronics can be easily integrated with biological systems for applications, including sensing physiological signals, diagnosing medical problems, opening the blood-brain barrier, and stimulating healing or tissue growth. Therefore, the generation of piezoelectricity from natural and synthetic bioresorbable polymers has drawn great attention in the research field. Herein, the significant and recent advancements in biodegradable piezoelectric materials, including natural and synthetic polymers, their principles, advanced applications, and challenges for medical uses, are reviewed thoroughly. The degradation methods of these piezoelectric materials through in vitro and in vivo studies are also investigated. These improvements in biodegradable piezoelectric materials and microsystems could enable new applications in the biomedical field. In the end, potential research opportunities regarding the practical applications are pointed out that might be significant for new materials research.
Keywords: biodegradables; biomedical devices; piezoelectric polymers.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Cui H., Hensleigh R., Yao D., Maurya D., Kumar P., Kang M. G., Priya S., Zheng X. R., Nat. Mater. 2019, 18, 234. - PubMed
-
- Malic B., Rojac T., Nat. Mater. 2018, 17, 297. - PubMed
-
- Chorsi M. T., Curry E. J., Chorsi H. T., Das R., Baroody J., Purohit P. K., Ilies H., Nguyen T. D., Adv. Mater. 2019, 31, 1802084. - PubMed
-
- Ramadan K. S., Sameoto D., Evoy S., Smart Mater. Struct. 2014, 23, 033001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

