A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases
- PMID: 37236155
- PMCID: PMC10228754
- DOI: 10.1016/j.cell.2023.04.025
A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases
Abstract
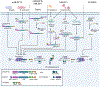
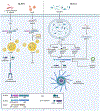
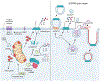
Inflammasomes are critical sentinels of the innate immune system that respond to threats to the host through recognition of distinct molecules, known as pathogen- or damage-associated molecular patterns (PAMPs/DAMPs), or disruptions of cellular homeostasis, referred to as homeostasis-altering molecular processes (HAMPs) or effector-triggered immunity (ETI). Several distinct proteins nucleate inflammasomes, including NLRP1, CARD8, NLRP3, NLRP6, NLRC4/NAIP, AIM2, pyrin, and caspases-4/-5/-11. This diverse array of sensors strengthens the inflammasome response through redundancy and plasticity. Here, we present an overview of these pathways, outlining the mechanisms of inflammasome formation, subcellular regulation, and pyroptosis, and discuss the wide-reaching effects of inflammasomes in human disease.
Keywords: AIM2; CARD8; GSDMD; IL-18; IL-1β; NLRC4; NLRP1; NLRP3; NLRP6; caspase-1; caspase-11; caspase-4; caspase-5; inflammasome; pyrin; pyroptosis.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.P.-Y.T. is a cofounder of IMMvention Therapeutix that works on inflammasome inhibitors.
Figures


References
-
- Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, and Bertin J (2002). PYPAF7, a Novel PYRIN-containing Apaf1-like Protein That Regulates Activation of NF-κB and Caspase-1-dependent Cytokine Processing*. J Biol Chem 277, 29874–29880. 10.1074/jbc.m203915200. - DOI - PubMed
-
- Koonin EV, and Aravind L (2000). The NACHT family - a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci 25, 223–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

