Memory profiles distinguish cross-reactive and virus-specific T cell immunity to mpox
- PMID: 37236191
- PMCID: PMC10211501
- DOI: 10.1016/j.chom.2023.04.015
Memory profiles distinguish cross-reactive and virus-specific T cell immunity to mpox
Abstract
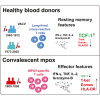
Mpox represents a persistent health concern with varying disease severity. Reinfections with mpox virus (MPXV) are rare, possibly indicating effective memory responses to MPXV or related poxviruses, notably vaccinia virus (VACV) from smallpox vaccination. We assessed cross-reactive and virus-specific CD4+ and CD8+ T cells in healthy individuals and mpox convalescent donors. Cross-reactive T cells were most frequently observed in healthy donors over 45 years. Notably, long-lived memory CD8+ T cells targeting conserved VACV/MPXV epitopes were identified in older individuals more than four decades after VACV exposure and exhibited stem-like characteristics, defined by T cell factor-1 (TCF-1) expression. In mpox convalescent donors, MPXV-reactive CD4+ and CD8+ T cells were more prevalent than in controls, demonstrating enhanced functionality and skewing toward effector phenotypes, which correlated with milder disease. Collectively, we report robust effector memory MPXV-specific T cell responses in mild mpox and long-lived TCF-1+ VACV/MPXV-specific CD8+ T cells decades after smallpox vaccination.
Keywords: MPXV; T cells; VACV; infectious diseases; long-lived memory T cells; monkeypox; mpox; vaccinia virus; viral immunity.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.B. is a consultant for Oxford Immunotec, Mabtech, Bristol-Myers Squibb, and MSD.
Figures




References
-
- WHO WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. 2022. https://www.who.int/europe/news/item/23-07-2022-who-director-general-dec...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

