Neuronally Derived Soluble Abeta Evokes Cell-Wide Astrocytic Calcium Dysregulation in Absence of Amyloid Plaques in Vivo
- PMID: 37236808
- PMCID: PMC10312057
- DOI: 10.1523/JNEUROSCI.1988-22.2023
Neuronally Derived Soluble Abeta Evokes Cell-Wide Astrocytic Calcium Dysregulation in Absence of Amyloid Plaques in Vivo
Abstract
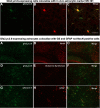
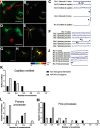
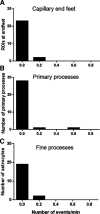
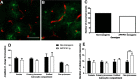
The key pathologic entities driving the destruction of synaptic function and integrity during the evolution of Alzheimer's disease (AD) remain elusive. Astrocytes are structurally and functionally integrated within synaptic and vascular circuitry and use calcium-based physiology to modulate basal synaptic transmission, vascular dynamics, and neurovascular coupling, which are central to AD pathogenesis. We used high-resolution multiphoton imaging to quantify all endogenous calcium signaling arising spontaneously throughout astrocytic somata, primary processes, fine processes, and capillary endfeet in the brain of awake APP/PS1 transgenic mice (11 male and 6 female mice). Endogenous calcium signaling within capillary endfeet, while surprisingly as active as astrocytic fine processes, was reduced ∼50% in the brain of awake APP/PS1 mice. Cortical astrocytes, in the presence of amyloid plaques in awake APP/PS1 mice, had a cell-wide increase in intracellular calcium associated with an increased frequency, amplitude, and duration of spontaneous calcium signaling. The cell-wide astrocytic calcium dysregulation was not directly related to distance to amyloid plaques. We could re-create the cell-wide intracellular calcium dysregulation in the absence of amyloid plaques following acute exposure to neuronally derived soluble Abeta from Tg2576 transgenic mice, in the living brain of male C57/Bl6 mice. Our findings highlight a role for astrocytic calcium pathophysiology in soluble-Abeta mediated neurodegenerative processes in AD. Additionally, therapeutic strategies aiming to protect astrocytic calcium physiology from soluble Abeta-mediated toxicity may need to pharmacologically enhance calcium signaling within the hypoactive capillary endfeet while reducing the hyperactivity of spontaneous calcium signaling throughout the rest of the astrocyte.SIGNIFICANCE STATEMENT Astrocytic calcium signaling is functionally involved in central pathologic processes of Alzheimer's disease. We quantified endogenous calcium signaling arising spontaneously in the brain of awake APP/PS1 mice, as general anesthesia suppressed astrocytic calcium signaling. Cell-wide astrocytic calcium dysregulation was not related to distance to amyloid plaques but mediated in part by neuronally derived soluble Abeta, supporting a role for astrocytes in soluble-Abeta mediated neurodegeneration. Spontaneous calcium signaling is largely compartmentalized and capillary endfeet were as active as fine processes but hypoactive in the presence of amyloid plaques, while the rest of the astrocyte became hyperactive. The cell-wide calcium pathophysiology in astrocytes may require a combination therapeutic strategy for hypoactive endfeet and astrocytic hyperactivity.
Keywords: Abeta; Alzheimer's disease; astrocytes; calcium signaling; in vivo imaging.
Copyright © 2023 the authors.
Figures








References
-
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H 3rd, Kraner SD, Norris CM (2009) Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci 29:12957–12969. 10.1523/JNEUROSCI.1064-09.2009 - DOI - PMC - PubMed
-
- Arbel-Ornath M, Hudry E, Boivin JR, Hashimoto T, Takeda S, Kuchibhotla KV, Hou S, Lattarulo CR, Belcher AM, Shakerdge N, Trujillo PB, Muzikansky A, Betensky RA, Hyman BT, Bacskai BJ (2017) Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol Neurodegener 12:27. 10.1186/s13024-017-0169-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
