Deep learning-based electrocardiographic screening for chronic kidney disease
- PMID: 37237055
- PMCID: PMC10220039
- DOI: 10.1038/s43856-023-00278-w
Deep learning-based electrocardiographic screening for chronic kidney disease
Abstract
Background: Undiagnosed chronic kidney disease (CKD) is a common and usually asymptomatic disorder that causes a high burden of morbidity and early mortality worldwide. We developed a deep learning model for CKD screening from routinely acquired ECGs.
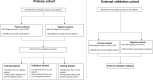
Methods: We collected data from a primary cohort with 111,370 patients which had 247,655 ECGs between 2005 and 2019. Using this data, we developed, trained, validated, and tested a deep learning model to predict whether an ECG was taken within one year of the patient receiving a CKD diagnosis. The model was additionally validated using an external cohort from another healthcare system which had 312,145 patients with 896,620 ECGs between 2005 and 2018.
Results: Using 12-lead ECG waveforms, our deep learning algorithm achieves discrimination for CKD of any stage with an AUC of 0.767 (95% CI 0.760-0.773) in a held-out test set and an AUC of 0.709 (0.708-0.710) in the external cohort. Our 12-lead ECG-based model performance is consistent across the severity of CKD, with an AUC of 0.753 (0.735-0.770) for mild CKD, AUC of 0.759 (0.750-0.767) for moderate-severe CKD, and an AUC of 0.783 (0.773-0.793) for ESRD. In patients under 60 years old, our model achieves high performance in detecting any stage CKD with both 12-lead (AUC 0.843 [0.836-0.852]) and 1-lead ECG waveform (0.824 [0.815-0.832]).
Conclusions: Our deep learning algorithm is able to detect CKD using ECG waveforms, with stronger performance in younger patients and more severe CKD stages. This ECG algorithm has the potential to augment screening for CKD.
Plain language summary
Chronic kidney disease (CKD) is a common condition involving loss of kidney function over time and results in a substantial number of deaths. However, CKD often has no symptoms during its early stages. To detect CKD earlier, we developed a computational approach for CKD screening using routinely acquired electrocardiograms (ECGs), a cheap, rapid, non-invasive, and commonly obtained test of the heart’s electrical activity. Our model achieved good accuracy in identifying any stage of CKD, with especially high accuracy in younger patients and more severe stages of CKD. Given the high global burden of undiagnosed CKD, novel and accessible CKD screening strategies have the potential to help prevent disease progression and reduce premature deaths related to CKD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Chronic Kidney Disease Prognosis C, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. - DOI - PMC - PubMed

