Adipsin alleviates cardiac microvascular injury in diabetic cardiomyopathy through Csk-dependent signaling mechanism
- PMID: 37237266
- PMCID: PMC10224320
- DOI: 10.1186/s12916-023-02887-7
Adipsin alleviates cardiac microvascular injury in diabetic cardiomyopathy through Csk-dependent signaling mechanism
Abstract
Background: Microvascular complications are associated with an overtly increased risk of adverse outcomes in patients with diabetes including coronary microvascular injury which manifested as disruption of adherens junctions between cardiac microvascular endothelial cells (CMECs). However, particular mechanism leading to diabetic coronary microvascular hyperpermeability remains elusive.
Methods: Experimental diabetes was induced in mice with adipose tissue-specific Adipsin overexpression (AdipsinLSL/LSL-Cre) and their respective control (AdipsinLSL/LSL). In addition, cultured CMECs were subjected to high glucose/palmitic acid (HG + PA) treatment to simulate diabetes for a mechanistic approach.
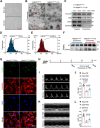
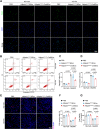
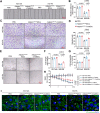
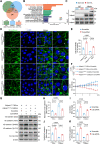
Results: The results showed that Adipsin overexpression significantly reduced cardiac microvascular permeability, preserved coronary microvascular integrity, and increased coronary microvascular density. Adipsin overexpression also attenuated cardiac dysfunction in diabetic mice. E/A ratio, an indicator of cardiac diastolic function, was improved by Adipsin. Adipsin overexpression retarded left ventricular adverse remodeling, enhanced LVEF, and improved cardiac systolic function. Adipsin-enriched exosomes were taken up by CMECs, inhibited CMECs apoptosis, and increased CMECs proliferation under HG + PA treatment. Adipsin-enriched exosomes also accelerated wound healing, rescued cell migration defects, and promoted tube formation in response to HG + PA challenge. Furthermore, Adipsin-enriched exosomes maintained adherens junctions at endothelial cell borders and reversed endothelial hyperpermeability disrupted by HG + PA insult. Mechanistically, Adipsin blocked HG + PA-induced Src phosphorylation (Tyr416), VE-cadherin phosphorylation (Tyr685 and Tyr731), and VE-cadherin internalization, thus maintaining CMECs adherens junctions integrity. LC-MS/MS analysis and co-immunoprecipitation analysis (Co-IP) unveiled Csk as a direct downstream regulator of Adipsin. Csk knockdown increased Src phosphorylation (Tyr416) and VE-cadherin phosphorylation (Tyr685 and Tyr731), while abolishing Adipsin-induced inhibition of VE-cadherin internalization. Furthermore, Csk knockdown counteracted Adipsin-induced protective effects on endothelial hyperpermeability in vitro and endothelial barrier integrity of coronary microvessels in vivo.
Conclusions: Together, these findings favor the vital role of Adipsin in the regulation of CMECs adherens junctions integrity, revealing its promises as a treatment target against diabetic coronary microvascular dysfunction. Graphical abstract depicting the mechanisms of action behind Adipsin-induced regulation of diabetic coronary microvascular dysfunction.
Trial registration: ClinicalTrials.gov NCT04570527.
Keywords: Adherens junctions; Adipsin; Diabetic cardiomyopathy; Exosomes; Permeability.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9. - DOI - PMC - PubMed
-
- Kristensen SL, Rørth R, Jhund PS, Shen L, Lee M, Petrie MC, et al. Microvascular complications in diabetes patients with heart failure and reduced ejection fraction-insights from the Beta-blocker evaluation of survival trial. Eur J Heart Fail. 2018;20(11):1549–56. doi: 10.1002/ejhf.1201. - DOI - PubMed
-
- Solini A, Penno G, Bonora E, Fondelli C, Orsi E, Arosio M, et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2012;35(1):143–9. doi: 10.2337/dc11-1380. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

