Estrogen modulation of cortical spreading depression
- PMID: 37237336
- PMCID: PMC10214707
- DOI: 10.1186/s10194-023-01598-x
Estrogen modulation of cortical spreading depression
Abstract
Background and aims: Cortical spreading depression (CSD), a transient neuronal and glial depolarization that propagates slowly across the cerebral cortex, is the putative electrophysiological event underlying migraine aura and a headache trigger. Migraine is three times more prevalent in women than men, linked to circulating female hormones. High estrogen levels or estrogen withdrawal may be a migraine trigger for many women. We, therefore, aimed to examine whether sex, gonadectomy, and female hormone supplementation and withdrawal affect the susceptibility to CSD.
Methods: To determine CSD susceptibility, we recorded the frequency of CSDs triggered during 2-h topical KCl application in intact or gonadectomized female and male rats, without or with estradiol or progesterone supplementation via daily intraperitoneal injections. Estrogen or progesterone treatment followed by withdrawal was studied in a separate cohort. To take the first step towards identifying potential mechanisms, we studied glutamate and GABAA receptor binding using autoradiography.
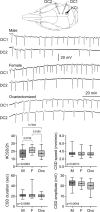
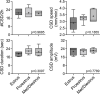
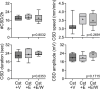
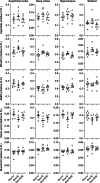
Results: The CSD frequency in intact female rats was higher than intact male and ovariectomized rats. We did not detect a change in CSD frequency during different stages of the estrous cycle in intact females. Daily estrogen injections for three weeks did not change CSD frequency. However, one-week estrogen withdrawal after two weeks of treatment significantly increased CSD frequency compared with the vehicle group in gonadectomized females. The same protocol of estrogen treatment and withdrawal was ineffective in gonadectomized males. In contrast to estrogen, daily progesterone injections for three weeks elevated CSD susceptibility, and one-week withdrawal after two weeks of treatment partially normalized this effect. Autoradiography did not reveal significant changes in glutamate or GABAA receptor binding density after estrogen treatment and withdrawal.
Conclusions: These data suggest that females are more susceptible to CSD, and sexual dimorphism is abrogated by gonadectomy. Moreover, estrogen withdrawal after prolonged daily treatment enhances CSD susceptibility. These findings may have implications for estrogen-withdrawal migraine, although the latter tends to be without aura.
Keywords: Aura; Cortical spreading depression; Estrogen replacement; Gonadal hormones; Migraine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

