Tumor Microenvironment and Glioblastoma Cell Interplay as Promoters of Therapeutic Resistance
- PMID: 37237548
- PMCID: PMC10215375
- DOI: 10.3390/biology12050736
Tumor Microenvironment and Glioblastoma Cell Interplay as Promoters of Therapeutic Resistance
Abstract
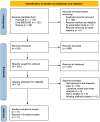
The invasive nature of glioblastoma is problematic in a radical surgery approach and can be responsible for tumor recurrence. In order to create new therapeutic strategies, it is imperative to have a better understanding of the mechanisms behind tumor growth and invasion. The continuous cross-talk between glioma stem cells (GSCs) and the tumor microenvironment (TME) contributes to disease progression, which renders research in this field difficult and challenging. The main aim of the review was to assess the different possible mechanisms that could explain resistance to treatment promoted by TME and GSCs in glioblastoma, including the role of M2 macrophages, micro RNAs (miRNAs), and long non-coding RNAs (lncRNAs) from exosomes from the TME. A systematic review of the literature on the role of the TME in developing and promoting radioresistance and chemoresistance of GBM was performed according to PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) guidelines. A dedicated literature review search was also performed on the immunotherapeutic agents against the immune TME. We identified 367 papers using the reported keywords. The final qualitative analysis was conducted on 25 studies. A growing amount of evidence in the current literature supports the role of M2 macrophages and non-coding RNAs in promoting the mechanisms of chemo and radioresistance. A better insight into how GBM cells interact with TME is an essential step towards comprehending the mechanisms that give rise to resistance to standard treatment, which can help to pave the way for the development of novel therapeutic strategies for GBM patients.
Keywords: M2-macrophages; chemoresistance; immunotherapy; non-coding RNA; radioresistance; stem cells; temozolomide; tumor microenvironment; tumor-associated macrophages.
Conflict of interest statement
The authors have no conflict of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report.
Figures



References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Ius T., Pignotti F., Della Pepa G.M., La Rocca G., Somma T., Isola M., Battistella C., Gaudino S., Polano M., Dal Bo M., et al. A Novel Comprehensive Clinical Stratification Model to Refine Prognosis of Glioblastoma Patients Undergoing Surgical Resection. Cancers. 2020;12:386. doi: 10.3390/cancers12020386. - DOI - PMC - PubMed
-
- Ius T., Somma T., Altieri R., Angileri F.F., Barbagallo G.M., Cappabianca P., Certo F., Cofano F., D’Elia A., Della Pepa G.M., et al. Is age an additional factor in the treatment of elderly patients with glioblastoma? A new stratification model: An Italian Multicenter Study. Neurosurg. Focus. 2020;49:E13. doi: 10.3171/2020.7.FOCUS20420. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

