Characterization and Homology Modeling of Catalytically Active Recombinant PhaCAp Protein from Arthrospira platensis
- PMID: 37237563
- PMCID: PMC10215190
- DOI: 10.3390/biology12050751
Characterization and Homology Modeling of Catalytically Active Recombinant PhaCAp Protein from Arthrospira platensis
Abstract
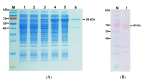
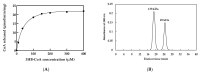
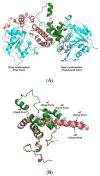
Polyhydroxybutyrate (PHB) is a biocompatible and biodegradable polymer that has the potential to replace fossil-derived polymers. The enzymes involved in the biosynthesis of PHB are β-ketothiolase (PhaA), acetoacetyl-CoA reductase (PhaB), and PHA synthase (PhaC). PhaC in Arthrospira platensis is the key enzyme for PHB production. In this study, the recombinant E. cloni®10G cells harboring A. platensis phaC (rPhaCAp) was constructed. The overexpressed and purified rPhaCAp with a predicted molecular mass of 69 kDa exhibited Vmax, Km, and kcat values of 24.5 ± 2 μmol/min/mg, 31.3 ± 2 µM and 412.7 ± 2 1/s, respectively. The catalytically active rPhaCAp was a homodimer. The three-dimensional structural model for the asymmetric PhaCAp homodimer was constructed based on Chromobacterium sp. USM2 PhaC (PhaCCs). The obtained model of PhaCAp revealed that the overall fold of one monomer was in the closed, catalytically inactive conformation whereas the other monomer was in the catalytically active, open conformation. In the active conformation, the catalytic triad residues (Cys151-Asp310-His339) were involved in the binding of substrate 3HB-CoA and the CAP domain of PhaCAp involved in the dimerization.
Keywords: 3HB-CoA; Arthrospira platensis; PHA synthase; PhaC; Polyhydroxybutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Price S., Kuzhiumparambil U., Pernice M., Ralph P. Techno-economic analysis of cyanobacterial PHB bioplastic production. J. Environ. Chem. Eng. 2022;10:107502. doi: 10.1016/j.jece.2022.107502. - DOI
-
- Alves A.A., Siqueira E.C., Barros M.P.S., Silva P.E.C., Houllou L.M. Polyhydroxyalkanoates: A review of microbial production and technology application. Int. J. Environ. Sci. Technol. 2023;20:3409–3420. doi: 10.1007/s13762-022-04213-9. - DOI
-
- Sharma S., Sharma P., Sharma V., Bajaj B.K. Polyhydroxybutyrate as an eco-friendly alternative of synthetic plastics. In: Mishra B.B., Nayak S.K., Mohapatra S., Samantaray D., editors. Environmental and Agricultural Microbiology: Applications for Sustainability. Springer; Berlin/Heidelberg, Germany: 2021. pp. 101–149.
-
- Madbouly S.A. 8 Bio-based polyhydroxyalkanoates blends and composites. In: Madbouly S.A., Zhang C., editors. Biopolymers and Composites: Processing and Characterization. Boston; Berlin, Germany: 2021. pp. 235–254.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

