A Low-Cost Fertilizer Medium Supplemented with Urea for the Lutein Production of Chlorella sp. and the Ability of the Lutein to Protect Cells against Blue Light Irradiation
- PMID: 37237664
- PMCID: PMC10215514
- DOI: 10.3390/bioengineering10050594
A Low-Cost Fertilizer Medium Supplemented with Urea for the Lutein Production of Chlorella sp. and the Ability of the Lutein to Protect Cells against Blue Light Irradiation
Abstract
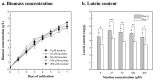
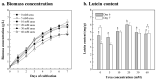
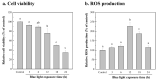
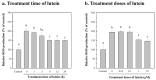
This study aimed to investigate the use of organic fertilizers instead of modified f/2 medium for Chlorella sp. cultivation, and the extracted lutein of the microalga to protect mammal cells against blue-light irradiation. The biomass productivity and lutein content of Chlorella sp. cultured in 20 g/L fertilizer medium for 6 days were 1.04 g/L/d and 4.41 mg/g, respectively. These values are approximately 1.3- and 1.4-fold higher than those achieved with the modified f/2 medium, respectively. The cost of medium per gram of microalgal biomass reduced by about 97%. The microalgal lutein content was further increased to 6.03 mg/g in 20 g/L fertilizer medium when supplemented with 20 mM urea, and the cost of medium per gram lutein reduced by about 96%. When doses of ≥1 μM microalgal lutein were used to protect mammal NIH/3T3 cells, there was a significant reduction in the levels of reactive oxygen species (ROS) produced by the cells in the following blue-light irradiation treatments. The results show that microalgal lutein produced by fertilizers with urea supplements has the potential to develop anti-blue-light oxidation products and reduce the economic challenges of microalgal biomass applied to carbon biofixation and biofuel production.
Keywords: Chlorella; ROS; blue light; fertilizer; lutein; urea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jaiswal K.K., Dutta S., Banerjee I., Pohrmen C.B., Kumar V. Photosynthetic microalgae-based carbon sequestration and generation of biomass in biorefinery approach for renewable biofuels for a cleaner environment. Biomass Convers. Biorefinery. 2021;11:1–19. doi: 10.1007/s13399-021-01504-y. - DOI
-
- Bibi R., Ahmad Z., Imran M., Hussain S., Ditta A., Mahmood S., Khalid A. Algal bioethanol production technology: A trend towards sustainable development. Renew. Sustain. Energy Rev. 2017;71:976–985. doi: 10.1016/j.rser.2016.12.126. - DOI
-
- Cuevas-Castillo G.A., Navarro-Pineda F.S., Rodríguez S.A.B., Rivero J.C.S. Advances on the processing of microalgal biomass for energy-driven biorefineries. Renew. Sustain. Energy Rev. 2020;125:109606. doi: 10.1016/j.rser.2019.109606. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

