Evaluation of Dosing Guidelines for Gentamicin in Neonates and Children
- PMID: 37237713
- PMCID: PMC10215193
- DOI: 10.3390/antibiotics12050810
Evaluation of Dosing Guidelines for Gentamicin in Neonates and Children
Abstract
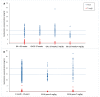
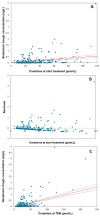
Although aminoglycosides are frequently prescribed to neonates and children, the ability to reach effective and safe target concentrations with the currently used dosing regimens remains unclear. This study aims to evaluate the target attainment of the currently used dosing regimens for gentamicin in neonates and children. We conducted a retrospective single-center cohort study in neonates and children receiving gentamicin between January 2019 and July 2022, in the Beatrix Children's Hospital. The first gentamicin concentration used for therapeutic drug monitoring was collected for each patient, in conjunction with information on dosing and clinical status. Target trough concentrations were ≤1 mg/L for neonates and ≤0.5 mg/L for children. Target peak concentrations were 8-12 mg/L for neonates and 15-20 mg/L for children. In total, 658 patients were included (335 neonates and 323 children). Trough concentrations were outside the target range in 46.2% and 9.9% of neonates and children, respectively. Peak concentrations were outside the target range in 46.0% and 68.7% of neonates and children, respectively. In children, higher creatinine concentrations were associated with higher gentamicin trough concentrations. This study corroborates earlier observational studies showing that, with a standard dose, drug concentration targets were met in only approximately 50% of the cases. Our findings show that additional parameters are needed to improve target attainment.
Keywords: children; dosage regimen; gentamicin; neonates; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kinderformularium Gentamicine. [(accessed on 30 November 2022)]. Available online: https://www.kinderformularium.nl/geneesmiddel/23/gentamicine.
LinkOut - more resources
Full Text Sources
Miscellaneous

