Carvacrol-A Natural Phenolic Compound with Antimicrobial Properties
- PMID: 37237727
- PMCID: PMC10215463
- DOI: 10.3390/antibiotics12050824
Carvacrol-A Natural Phenolic Compound with Antimicrobial Properties
Abstract
The main purpose of this article is to present the latest research related to selected biological properties of carvacrol, such as antimicrobial, anti-inflammatory, and antioxidant activity. As a monoterpenoid phenol, carvacrol is a component of many essential oils and is usually found in plants together with its isomer, thymol. Carvacrol, either alone or in combination with other compounds, has a strong antimicrobial effect on many different strains of bacteria and fungi that are dangerous to humans or can cause significant losses in the economy. Carvacrol also exerts strong anti-inflammatory properties by preventing the peroxidation of polyunsaturated fatty acids by inducing SOD, GPx, GR, and CAT, as well as reducing the level of pro-inflammatory cytokines in the body. It also affects the body's immune response generated by LPS. Carvacrol is considered a safe compound despite the limited amount of data on its metabolism in humans. This review also discusses the biotransformations of carvacrol, because the knowledge of the possible degradation pathways of this compound may help to minimize the risk of environmental contamination with phenolic compounds.
Keywords: antimicrobial; biotransformation; carvacrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
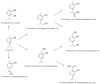
- Yadav G., Kamble S.B. Synthesis of carvacrol by Friedel–Crafts alkylation of o-cresol with isopropanol using superacidic catalyst UDCaT-5. J. Chem. Technol. Biotechnol. 2009;84:1499–1508. doi: 10.1002/jctb.2210. - DOI
-
- Marinelli L., di Stefano A., Cacciatore I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018;17:903–921. doi: 10.1007/s11101-018-9569-x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous