Aminoglycosides for the Treatment of Severe Infection Due to Resistant Gram-Negative Pathogens
- PMID: 37237763
- PMCID: PMC10215420
- DOI: 10.3390/antibiotics12050860
Aminoglycosides for the Treatment of Severe Infection Due to Resistant Gram-Negative Pathogens
Abstract
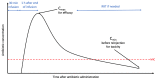
Aminoglycosides are a family of rapidly bactericidal antibiotics that often remain active against resistant Gram-negative bacterial infections. Over the past decade, their use in critically ill patients has been refined; however, due to their renal and cochleovestibular toxicity, their indications in the treatment of sepsis and septic shock have been gradually reduced. This article reviews the spectrum of activity, mode of action, and methods for optimizing the efficacy of aminoglycosides. We discuss the current indications for aminoglycosides, with an emphasis on multidrug-resistant Gram-negative bacteria, such as extended-spectrum β-lactamase-producing Enterobacterales, carbapenemase-producing Enterobacterales, multidrug-resistant Pseudomonas aeruginosa, and carbapenem-resistant Acinetobacter baumannii. Additionally, we review the evidence for the use of nebulized aminoglycosides.
Keywords: aminoglycosides; gram-negative infections; multidrug-resistant; nebulization; pharmacokinetics; toxicity.
Conflict of interest statement
Outside of the study, J.-F.T. declares participation to the boards of Merck, Pfizer, BD, Gilead, and Paratek, and lecture fees for Pfizer, Shionogi, BD, Biomerieux and Merck.
Figures
References
-
- Weinstein M.J., Luedemann G.M., Oden E.M., Wagman G.H. Gentamicin, A New Broad-Spectrum Antibiotic Complex. Antimicrob. Agents Chemother. 1963;161:1–7. - PubMed
-
- Kluge R.M., Standiford H.C., Tatem B., Young V.M., Greene W.H., Schimpff S.C., Calia F.M., Hornick R.B. Comparative Activity of Tobramycin, Amikacin, and Gentamicin Alone and with Carbenicillin against Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 1974;6:442–446. doi: 10.1128/AAC.6.4.442. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous