Distinct Roles of CK2- and AKT-Mediated NF-κB Phosphorylations in Clasmatodendrosis (Autophagic Astroglial Death) within the Hippocampus of Chronic Epilepsy Rats
- PMID: 37237886
- PMCID: PMC10215642
- DOI: 10.3390/antiox12051020
Distinct Roles of CK2- and AKT-Mediated NF-κB Phosphorylations in Clasmatodendrosis (Autophagic Astroglial Death) within the Hippocampus of Chronic Epilepsy Rats
Abstract
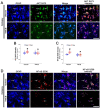
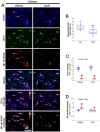
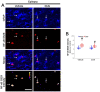
The downregulation of glutathione peroxidase-1 (GPx1) plays a role in clasmatodendrosis (an autophagic astroglial death) in the hippocampus of chronic epilepsy rats. Furthermore, N-acetylcysteine (NAC, a GSH precursor) restores GPx1 expression in clasmatodendritic astrocytes and alleviates this autophagic astroglial death, independent of nuclear factor erythroid-2-related factor 2 (Nrf2) activity. However, the regulatory signal pathways of these phenomena have not been fully explored. In the present study, NAC attenuated clasmatodendrosis by alleviating GPx1 downregulation, casein kinase 2 (CK2)-mediated nuclear factor-κB (NF-κB) serine (S) 529 and AKT-mediated NF-κB S536 phosphorylations. 2-[4,5,6,7-Tetrabromo-2-(dimethylamino)-1H-benzo[d]imidazole-1-yl]acetic acid (TMCB; a selective CK2 inhibitor) relieved clasmatodendritic degeneration and GPx1 downregulation concomitant with the decreased NF-κB S529 and AKT S473 phosphorylations. In contrast, AKT inhibition by 3-chloroacetyl-indole (3CAI) ameliorated clasmatodendrosis and NF-κB S536 phosphorylation, while it did not affect GPx1 downregulation and CK2 tyrosine (Y) 255 and NF-κB S529 phosphorylations. Therefore, these findings suggest that seizure-induced oxidative stress may diminish GPx1 expression by increasing CK2-mediated NF-κB S529 phosphorylation, which would subsequently enhance AKT-mediated NF-κB S536 phosphorylation leading to autophagic astroglial degeneration.
Keywords: 3CAI; GPx1; NAC; TMCB; astrocyte; autophagy; oxidative stress; seizure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Penfield W. Neuroglia and microglia—The interstitial tissue of the central nervous system. In: Cowdry E.V., editor. Special Cytology, the Form and Function of the Cell in Health and Disease. Hoeber; New York, NY, USA: 1928. pp. 1033–1068.
-
- Duchen L.W. General pathology of neurons and neuroglia. In: Adams J.H., Duchen L.W., editors. Greenfield’s Neuropathology. Oxford University Press; New York, NY, USA: 1992. pp. 1–68.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

