Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor
- PMID: 37237903
- PMCID: PMC10215290
- DOI: 10.3390/antiox12051037
Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor
Abstract
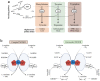
Tetrahydrobiopterin (BH4) is an endogenous cofactor for some enzymatic conversions of essential biomolecules, including nitric oxide, and monoamine neurotransmitters, and for the metabolism of phenylalanine and lipid esters. Over the last decade, BH4 metabolism has emerged as a promising metabolic target for negatively modulating toxic pathways that may result in cell death. Strong preclinical evidence has shown that BH4 metabolism has multiple biological roles beyond its traditional cofactor activity. We have shown that BH4 supports essential pathways, e.g., to generate energy, to enhance the antioxidant resistance of cells against stressful conditions, and to protect from sustained inflammation, among others. Therefore, BH4 should not be understood solely as an enzyme cofactor, but should instead be depicted as a cytoprotective pathway that is finely regulated by the interaction of three different metabolic pathways, thus assuring specific intracellular concentrations. Here, we bring state-of-the-art information about the dependency of mitochondrial activity upon the availability of BH4, as well as the cytoprotective pathways that are enhanced after BH4 exposure. We also bring evidence about the potential use of BH4 as a new pharmacological option for diseases in which mitochondrial disfunction has been implicated, including chronic metabolic disorders, neurodegenerative diseases, and primary mitochondriopathies.
Keywords: antioxidant; inflammation; memory; mitochondrial enhancer; neopterin; oxidative stress; sepiapterin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hirakawa H., Sawada H., Yamahama Y., Takikawa S.-I., Shintaku H., Hara A., Mase K., Kondo T., Iino T. Expression Analysis of the Aldo-Keto Reductases Involved in the Novel Biosynthetic Pathway of Tetrahydrobiopterin in Human and Mouse Tissues. J. Biochem. 2009;146:51–60. doi: 10.1093/jb/mvp042. - DOI - PubMed
-
- D’Sa C., Hirayama K., West A., Hahn M., Zhu M., Kapatos G. Tetrahydrobiopterin Biosynthesis in C6 Glioma Cells: Induction of GTP Cyclohydrolase I Gene Expression by Lipopolysaccharide and Cytokine Treatment. Brain Res. Mol. Brain Res. 1996;41:105–110. doi: 10.1016/0169-328X(96)00073-3. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

