Aucubin Exerts Neuroprotection against Forebrain Ischemia and Reperfusion Injury in Gerbils through Antioxidative and Neurotrophic Effects
- PMID: 37237948
- PMCID: PMC10215358
- DOI: 10.3390/antiox12051082
Aucubin Exerts Neuroprotection against Forebrain Ischemia and Reperfusion Injury in Gerbils through Antioxidative and Neurotrophic Effects
Abstract
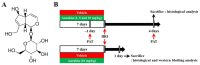
Aucubin is an iridoid glycoside that displays various pharmacological actions including antioxidant activity. However, there are few reports available on the neuroprotective effects of aucubin against ischemic brain injury. Thus, the aim of this study was to investigate whether aucubin protected against damage to hippocampal function induced by forebrain ischemia-reperfusion injury (fIRI) in gerbils, and to examine whether aucubin produced neuroprotection in the hippocampus against fIRI and to explore its mechanisms by histopathology, immunohistochemistry, and Western analysis. Gerbils were given intraperitoneal injections of aucubin at doses of 1, 5, and 10 mg/kg, respectively, once a day for seven days before fIRI. As assessed by the passive avoidance test, short-term memory function following fIRI significantly declined, whereas the decline in short-term memory function due to fIRI was ameliorated by pretreatment with 10 mg/kg, but not 1 or 5 mg/kg, of aucubin. Most of the pyramidal cells (principal cells) of the hippocampus died in the Cornu Ammonis 1 (CA1) area four days after fIRI. Treatment with 10 mg/kg, but not 1 or 5 mg/kg, of aucubin protected the pyramidal cells from IRI. The treatment with 10 mg/kg of aucubin significantly reduced IRI-induced superoxide anion production, oxidative DNA damage, and lipid peroxidation in the CA1 pyramidal cells. In addition, the aucubin treatment significantly increased the expressions of superoxide dismutases (SOD1 and SOD2) in the pyramidal cells before and after fIRI. Furthermore, the aucubin treatment significantly enhanced the protein expression levels of neurotrophic factors, such as brain-derived neurotrophic factor and insulin-like growth factor-I, in the hippocampal CA1 area before and after IRI. Collectively, in this experiment, pretreatment with aucubin protected CA1 pyramidal cells from forebrain IRI by attenuating oxidative stress and increasing neurotrophic factors. Thus, pretreatment with aucubin can be a promising candidate for preventing brain IRI.
Keywords: aucubin; hippocampus; histopathology; immunohistochemistry; oxidative stress; transient ischemia; western blotting.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Wen H., Liu L., Zhan L., Liang D., Li L., Liu D., Sun W., Xu E. Neuroglobin mediates neuroprotection of hypoxic postconditioning against transient global cerebral ischemia in rats through preserving the activity of na(+)/k(+) atpases. Cell Death Dis. 2018;9:635. doi: 10.1038/s41419-018-0656-0. - DOI - PMC - PubMed
-
- Globus M.Y., Busto R., Martinez E., Valdes I., Dietrich W.D., Ginsberg M.D. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J. Neurochem. 1991;57:470–478. doi: 10.1111/j.1471-4159.1991.tb03775.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

