Lignan-Rich Sesame (Sesamum indicum L.) Cultivar Exhibits In Vitro Anti-Cholinesterase Activity, Anti-Neurotoxicity in Amyloid-β Induced SH-SY5Y Cells, and Produces an In Vivo Nootropic Effect in Scopolamine-Induced Memory Impaired Mice
- PMID: 37237976
- PMCID: PMC10215706
- DOI: 10.3390/antiox12051110
Lignan-Rich Sesame (Sesamum indicum L.) Cultivar Exhibits In Vitro Anti-Cholinesterase Activity, Anti-Neurotoxicity in Amyloid-β Induced SH-SY5Y Cells, and Produces an In Vivo Nootropic Effect in Scopolamine-Induced Memory Impaired Mice
Abstract
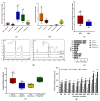
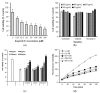
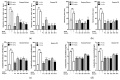
Alzheimer's disease, a major cause of dementia, is characterized by impaired cholinergic function, increased oxidative stress, and amyloid cascade induction. Sesame lignans have attracted considerable attention owing to their beneficial effects on brain health. This study investigated the neuroprotective potential of lignan-rich sesame cultivars. Among the 10 sesame varieties studied, Milyang 74 (M74) extracts exhibited the highest total lignan content (17.71 mg/g) and in vitro acetylcholinesterase (AChE) inhibitory activity (66.17%, 0.4 mg/mL). M74 extracts were the most effective in improving cell viability and inhibiting reactive oxygen species (ROS) and malondialdehyde (MDA) generation in amyloid-β25-35 fragment-treated SH-SY5Y cells. Thus, M74 was used to evaluate the nootropic effects of sesame extracts and oil on scopolamine (2 mg/kg)-induced memory impairment in mice compared to the control cultivar (Goenback). Pretreatment with the M74 extract (250 and 500 mg/kg) and oil (1 and 2 mL/kg) effectively improved memory disorder in mice (demonstrated by the passive avoidance test), inhibited AChE, and enhanced acetylcholine (Ach) levels. Moreover, immunohistochemistry and Western blot results showed that the M74 extract and oil reversed the scopolamine-induced increase in APP, BACE-1, and presenilin expression levels in the amyloid cascade and decreased BDNF and NGF expression levels in neuronal regeneration.
Keywords: amyloid-β; lignan; neuronal regeneration; neurotoxicity; sesame.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

