JAC4 Alleviates Rotenone-Induced Parkinson's Disease through the Inactivation of the NLRP3 Signal Pathway
- PMID: 37238000
- PMCID: PMC10215424
- DOI: 10.3390/antiox12051134
JAC4 Alleviates Rotenone-Induced Parkinson's Disease through the Inactivation of the NLRP3 Signal Pathway
Abstract
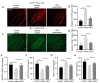
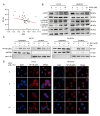
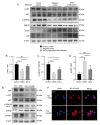
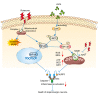
Parkinson's disease (PD) is the fastest-growing neurodegeneration disease, characterized typically by a progressive loss of dopaminergic neurons in the substantia nigra, and there are no effective therapeutic agents to cure PD. Rotenone (Rot) is a common and widely used pesticide which can directly inhibit mitochondrial complex I, leading to a loss of dopaminergic neurons. Our previous studies proved that the JWA gene (arl6ip5) may play a prominent role in resisting aging, oxidative stress and inflammation, and JWA knockout in astrocytes increases the susceptibility of mice to 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD. JWA-activating compound 4 (JAC4) is a small-molecule activator of the JWA gene, but its role in and mechanism against PD have not yet been clarified. In the present study, we showed that the JWA expression level is strongly related to tyrosine hydroxylase (TH) in different growth periods of mice. Additionally, we constructed models with Rot in vivo and in vitro to observe the neuroprotective effects of JAC4. Our results demonstrated that JAC4 prophylactic intervention improved motor dysfunction and dopaminergic neuron loss in mice. Mechanistically, JAC4 reduced oxidative stress damage by reversing mitochondrial complex I damage, reducing nuclear factor kappa-B (NF-κB) translocation and repressing nucleotide-binding domain, leucine-rich-containing family and pyrin domain-containing-3 (NLRP3) inflammasome activation. Overall, our results provide proof that JAC4 could serve as a novel effective agent for PD prevention.
Keywords: JAC4; NLRP3 inflammasome; Parkinson’s disease; mitochondrial complex I; oxidative stress; rotenone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

