Histopathological Study on Collagen in Full-Thickness Wound Healing in Fraser's Dolphins (Lagenodelphis hosei)
- PMID: 37238111
- PMCID: PMC10215821
- DOI: 10.3390/ani13101681
Histopathological Study on Collagen in Full-Thickness Wound Healing in Fraser's Dolphins (Lagenodelphis hosei)
Abstract
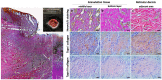
Fraser's dolphins (Lagenodelphis hosei) possess great healing abilities. Their skin composition can be restored after wounding, including collagen spacing, orientation, and bundle thickness. However, it remains unclear how collagens are involved in the wound-healing process and eventually regain normality in Fraser's dolphins. Learned from the other two scarless healing animals, changes in type III/I collagen composition are believed to modulate the wound healing process and influence the scarring or scarless fate determination in human fetal skin and spiny mouse skin. In the current study, Herovici's, trichrome, and immunofluorescence staining were used on normal and wounded skin samples in Fraser's dolphins. The results suggested that type I collagens were the main type of collagens in the normal skin of Fraser's dolphins, while type III collagens were barely seen. During the wound healing process, type III collagens showed at early wound healing stages, and type I collagen increased in the mature healed wound. In an early healed wound, collagens were organized in a parallel manner, showing a transient hypertrophic-like scar, and eventually restored to normal collagen configuration and adipocyte distribution in the mature healed wound. The remarkable ability to remove excessive collagens merits further investigation to provide new insights into clinical wound management.
Keywords: Fraser’s dolphins; type I collagen; type III collagen; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

