Painless Capillary Blood Collection: A Rapid Evaluation of the Onflow Device
- PMID: 37238237
- PMCID: PMC10217062
- DOI: 10.3390/diagnostics13101754
Painless Capillary Blood Collection: A Rapid Evaluation of the Onflow Device
Abstract
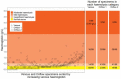
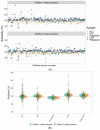
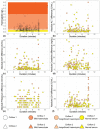
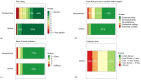
Blood-based diagnostics are critical for many medical decisions, but mostly rely on venepuncture, which can be inconvenient and painful. The Onflow Serum Gel (Loop Medical SA, Vaud, Lausanne, Switzerland) is a novel blood collection device that utilises needle-free technology to collect capillary blood. In this pilot study, 100 healthy participants were enrolled and provided two Onflow collected specimens and one venous blood specimen. Five chemistry analytes (AST, ALT, LDH, potassium, creatinine) and haemolysis were measured per specimen, and laboratory analyte results were compared. Onflow was found to be more acceptable than venepuncture with lower pain ratings, and 96.5% of participants would use the Onflow method again. All phlebotomists (100%) found Onflow intuitive and user-friendly, with ~1 mL of Onflow blood successfully collected from 99% of participants in <12 min (mean: 6 min, 40 s) and 91% collected on the first attempt. ALT and AST analytes showed no difference in performance, while creatinine generated a negative bias (-5.6 µmol/L), and increased variability was noted with potassium (3.6%CV) and LDH (6.7%CV), although none were clinically relevant. These differences may be due to 35% of Onflow collected specimens having "mild" haemolysis. Onflow is a promising alternative blood collection device that should now be evaluated in participants with expected abnormal chemistries and as an option for self-collection.
Keywords: capillary blood collection; onflow serum gel; pain-free; serum chemistry; upper arm collection device; venous-comparable blood specimen.
Conflict of interest statement
Arthur Queval is the founder and CEO of Loop Medical SA. Charlotte Trotter and Alison Moran are employees of Loop Medical SA. The other authors declare no conflicts of interest.
Figures

References
-
- Maass K.F., Barfield M.D., Ito M., James C.A., Kavetska O., Kozinn M., Kumar P., Lepak M., Leuthold L.A., Li W., et al. Leveraging patient-centric sampling for clinical drug development and decentralized clinical trials: Promise to reality. Clin. Transl. Sci. 2022;15:2785–2795. doi: 10.1111/cts.13411. - DOI - PMC - PubMed
-
- Ansari S., Abdel-Malek M., Kenkre J., Choudhury S.M., Barnes S., Misra S., Tan T., Cegla J. The use of whole blood capillary samples to measure 15 analytes for a home-collect biochemistry service during the SARS-CoV-2 pandemic: A proposed model from North West London Pathology. Ann. Clin. Biochem. 2021;58:411–421. doi: 10.1177/00045632211004995. - DOI - PMC - PubMed
-
- Knitza J., Tascilar K., Vuillerme N., Eimer E., Matusewicz P., Corte G., Schuster L., Aubourg T., Bendzuck G., Korinth M., et al. Accuracy and tolerability of self-sampling of capillary blood for analysis of inflammation and autoantibodies in rheumatoid arthritis patients-results from a randomized controlled trial. Arthritis Res. Ther. 2022;24:125. doi: 10.1186/s13075-022-02809-7. - DOI - PMC - PubMed
-
- Winnoz Inc. Haiim Vaccum Assisted Blood Collection System—Quick Guide for Operating. WH-QG-R-V2.0. 2021. [(accessed on 30 March 2023)]. Available online: https://drive.google.com/file/d/1T_mkqLaMRMR6RBky8ronR3OGCVmwYveK/view.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

