Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury
- PMID: 37238298
- PMCID: PMC10217493
- DOI: 10.3390/diagnostics13101814
Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury
Abstract
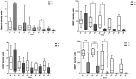
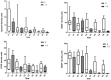
Acute traumatic spinal cord injury (SCI) is recognized as a global problem that can lead to a range of acute and secondary complications impacting morbidity and mortality. There is still a lack of reliable diagnostic and prognostic biomarkers in patients with SCI that could help guide clinical care and identify novel therapeutic targets for future drug discovery. The aim of this prospective controlled study was to determine the cerebral spinal fluid (CSF) and serum profiles of 10 biomarkers as indicators of SCI diagnosis, severity, and prognosis to aid in assessing appropriate treatment modalities. CSF and serum samples of 15 SCI and ten healthy participants were included in the study. The neurological assessments were scored on admission and at discharge from the hospital using the American Spinal Injury Association Impairment Score (AIS) grades. The CSF and serum concentrations of SBDP150, S100B, GFAP, NF-L, UCHL-1, Tau, and IL-6 were significantly higher in SCI patients when compared with the control group. The CSF GBDP 38/44K, UCHL-L1, S100B, GFAP, and Tau levels were significantly higher in the AIS A patients. This study demonstrated a strong correlation between biomarker levels in the diagnosis and injury severity of SCI but no association with short-term outcomes. Future prospective controlled studies need to be done to support the results of this study.
Keywords: ASIA impairment scale; American Spinal Injury Association; GFAP breakdown product; S100 calcium-binding protein-B; biomarker; diagnostic and prognostic markers; glial fibrillary acidic protein; injury severity; interleukin; neurofilament light chain; spinal cord injury; ubiquitin C-terminal hydrolase-L1; αII-spectrin breakdown product.
Conflict of interest statement
R.L.H. was a shareholder and employee of Banyan Biomarkers, and K.W.W. is a shareholder of Gryphon Bio Inc.
Figures



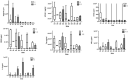


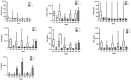
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

