Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein
- PMID: 37238850
- PMCID: PMC10217760
- DOI: 10.3390/foods12102030
Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein
Abstract
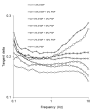
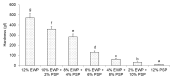
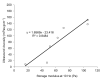
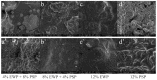
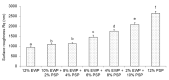
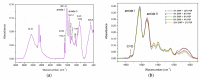
The aim of this study was to investigate the gelation process of binary mixes of pumpkin-seed and egg-white proteins. The substitution of pumpkin-seed proteins with egg-white proteins improved the rheological properties of the obtained gels, i.e., a higher storage modulus, lower tangent delta, and larger ultrasound viscosity and hardness. Gels with a larger egg-white protein content were more elastic and more resistant to breaking structure. A higher concentration of pumpkin-seed protein changed the gel microstructure to a rougher and more particulate one. The microstructure was less homogenous, with a tendency to break at the pumpkin/egg-white protein gel interface. The decrease in the intensity of the amide II band with an increase in the pumpkin-seed protein concentration showed that the secondary structure of this protein evolved more toward a linear amino acid chain compared with the egg-white protein, which could have an impact on the microstructure. The supplementation of pumpkin-seed proteins with egg-white proteins caused a decrease in water activity from 0.985 to 0.928, which had important implications for the microbiological stability of the obtained gels. Strong correlations were found between the water activity and rheological properties of the gels; an improvement of their rheological properties resulted in a decrease in water activity. The supplementation of pumpkin-seed proteins with egg-white proteins resulted in more homogenous gels with a stronger microstructure and better water binding.
Keywords: FTIR; egg white; gelation; microstructure; protein; pumpkin; rheology; texture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pérez-Huertas S., Terpiłowski K., Tomczyńska-Mleko M., Nishinari K., Mleko S. Surface and rheological properties of egg white albumin/gelatin dispersions gelled on cold plasma-activated glass. Food Hydrocoll. 2015;96:224–230. doi: 10.1016/j.foodhyd.2019.05.029. - DOI
-
- Weng Y., Sun Z. Major cucurbit crops. In: Wang Y.-H., Behera T.K., Kole C., editors. Genetics, Genomics and Breeding of Cucurbits. CRC Press; Boca Raton, FL, USA: 2012. pp. 1–16.
LinkOut - more resources
Full Text Sources

