Complexity of Sex Differences and Their Impact on Alzheimer's Disease
- PMID: 37238932
- PMCID: PMC10215447
- DOI: 10.3390/biomedicines11051261
Complexity of Sex Differences and Their Impact on Alzheimer's Disease
Abstract
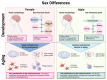
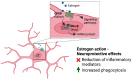
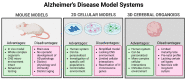
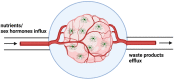
Sex differences are present in brain morphology, sex hormones, aging processes and immune responses. These differences need to be considered for proper modelling of neurological diseases with clear sex differences. This is the case for Alzheimer's disease (AD), a fatal neurodegenerative disorder with two-thirds of cases diagnosed in women. It is becoming clear that there is a complex interplay between the immune system, sex hormones and AD. Microglia are major players in the neuroinflammatory process occurring in AD and have been shown to be directly affected by sex hormones. However, many unanswered questions remain as the importance of including both sexes in research studies has only recently started receiving attention. In this review, we provide a summary of sex differences and their implications in AD, with a focus on microglia action. Furthermore, we discuss current available study models, including emerging complex microfluidic and 3D cellular models and their usefulness for studying hormonal effects in this disease.
Keywords: Alzheimer’s disease; cerebral organoids; estrogen; hiPSC; microglia; sex differences; sex hormones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Steinberg J.R., Turner B.E., Weeks B.T., Magnani C.J., Wong B.O., Rodriguez F., Yee L.M., Cullen M.R. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Netw. Open. 2021;4:e2113749. doi: 10.1001/jamanetworkopen.2021.13749. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

