Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm
- PMID: 37238945
- PMCID: PMC10215485
- DOI: 10.3390/biomedicines11051273
Targeted Proteomic Analysis of Patients with Ascending Thoracic Aortic Aneurysm
Abstract
Background: There is a need for clinical markers to aid in the detection of individuals at risk of harboring an ascending thoracic aneurysm (ATAA) or developing one in the future.
Objectives: To our knowledge, ATAA remains without a specific biomarker. This study aims to identify potential biomarkers for ATAA using targeted proteomic analysis.
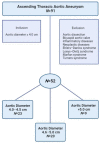
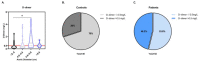
Methods: In this study, 52 patients were divided into three groups depending on their ascending aorta diameter: 4.0-4.5 cm (N = 23), 4.6-5.0 cm (N = 20), and >5.0 cm (N = 9). A total of 30 controls were in-house populations ethnically matched to cases without known or visible ATAA-related symptoms and with no ATAA familial history. Before the debut of our study, all patients provided medical history and underwent physical examination. Diagnosis was confirmed by echocardiography and angio-computed tomography (CT) scans. Targeted-proteomic analysis was conducted to identify possible biomarkers for the diagnosis of ATAA.
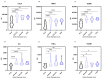
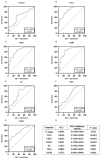
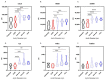
Results: A Kruskal-Wallis test revealed that C-C motif chemokine ligand 5 (CCL5), defensin beta 1 (HBD1), intracellular adhesion molecule-1 (ICAM1), interleukin-8 (IL8), tumor necrosis factor alpha (TNFα) and transforming growth factor-beta 1 (TGFB1) expressions are significantly increased in ATAA patients in comparison to control subjects with physiological aorta diameter (p < 0.0001). The receiver-operating characteristic analysis showed that the area under the curve values for CCL5 (0.84), HBD1 (0.83) and ICAM1 (0.83) were superior to that of the other analyzed proteins.
Conclusions: CCL5, HBD1 and ICAM1 are very promising biomarkers with satisfying sensitivity and specificity that could be helpful in stratifying risk for the development of ATAA. These biomarkers may assist in the diagnosis and follow-up of patients at risk of developing ATAA. This retrospective study is very encouraging; however, further in-depth studies may be worthwhile to investigate the role of these biomarkers in the pathogenesis of ATAA.
Keywords: C-C motif chemokine ligand 5; ascending aortic thoracic aneurysm; biomarkers; defensin beta 1; intracellular adhesion molecule-1; proteomics.
Conflict of interest statement
Authors Angeliki Minia, Vaia Pliaka and Leonidas G. Alexopoulos were employed by the company ProtATonce Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Isselbacher E.M., Preventza O., Hamilton B.I.J., Augoustides J.G., Beck A.W., Bolen M.A., Braverman A.C., Bray B.E., Brown-Zimmerman M.M., Chen E.P. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022;80:e223–e393. doi: 10.1016/j.jacc.2022.08.004. - DOI - PMC - PubMed
-
- Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., De Ferranti S., Després J.P., Fullerton H.J., Howard V.J., et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

