GM1 Oligosaccharide Efficacy in Parkinson's Disease: Protection against MPTP
- PMID: 37238977
- PMCID: PMC10216367
- DOI: 10.3390/biomedicines11051305
GM1 Oligosaccharide Efficacy in Parkinson's Disease: Protection against MPTP
Abstract
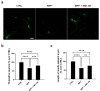
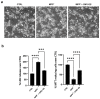
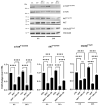
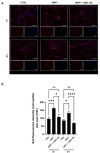
Past evidence has shown that the exogenous administration of GM1 ganglioside slowed neuronal death in preclinical models of Parkinson's disease, a neurodegenerative disorder characterized by the progressive loss of dopamine-producing neurons: however, the physical and chemical properties of GM1 (i.e., amphiphilicity) limited its clinical application, as the crossing of the blood-brain barrier is denied. Recently, we demonstrated that the GM1 oligosaccharide head group (GM1-OS) is the GM1 bioactive portion that, interacting with the TrkA-NGF complex at the membrane surface, promotes the activation of a multivariate network of intracellular events regulating neuronal differentiation, protection, and reparation. Here, we evaluated the GM1-OS neuroprotective potential against the Parkinson's disease-linked neurotoxin MPTP, which destroys dopaminergic neurons by affecting mitochondrial bioenergetics and causing ROS overproduction. In dopaminergic and glutamatergic primary cultures, GM1-OS administration significantly increased neuronal survival, preserved neurite network, and reduced mitochondrial ROS production enhancing the mTOR/Akt/GSK3β pathway. These data highlight the neuroprotective efficacy of GM1-OS in parkinsonian models through the implementation of mitochondrial function and reduction in oxidative stress.
Keywords: GM1 ganglioside; GM1 oligosaccharide; MPTP; Parkinson’s disease; neuroprotection; plasma membrane signaling.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
GM1 oligosaccharide efficacy against α-synuclein aggregation and toxicity in vitro.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Sep;1868(9):159350. doi: 10.1016/j.bbalip.2023.159350. Epub 2023 Jun 16. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 37330108 Free PMC article.
-
The oligosaccharide portion of ganglioside GM1 regulates mitochondrial function in neuroblastoma cells.Glycoconj J. 2020 Jun;37(3):293-306. doi: 10.1007/s10719-020-09920-4. Epub 2020 Apr 8. Glycoconj J. 2020. PMID: 32266604
-
Gangliosides in the differentiation process of primary neurons: the specific role of GM1-oligosaccharide.Glycoconj J. 2020 Jun;37(3):329-343. doi: 10.1007/s10719-020-09919-x. Epub 2020 Mar 20. Glycoconj J. 2020. PMID: 32198666
-
Turning the spotlight on the oligosaccharide chain of GM1 ganglioside.Glycoconj J. 2021 Feb;38(1):101-117. doi: 10.1007/s10719-021-09974-y. Epub 2021 Feb 23. Glycoconj J. 2021. PMID: 33620588 Free PMC article. Review.
-
The relationship between depletion of brain GM1 ganglioside and Parkinson's disease.FEBS Open Bio. 2023 Sep;13(9):1548-1557. doi: 10.1002/2211-5463.13554. Epub 2023 Feb 5. FEBS Open Bio. 2023. PMID: 36638010 Free PMC article. Review.
Cited by
-
GM1 Oligosaccharide Ameliorates Rett Syndrome Phenotypes In Vitro and In Vivo via Trk Receptor Activation.Int J Mol Sci. 2024 Oct 28;25(21):11555. doi: 10.3390/ijms252111555. Int J Mol Sci. 2024. PMID: 39519108 Free PMC article.
-
Lysosomal free sialic acid storage disorder iPSC-derived neural cells display altered glycosphingolipid metabolism.Sci Rep. 2025 Aug 13;15(1):29708. doi: 10.1038/s41598-025-12682-4. Sci Rep. 2025. PMID: 40804080 Free PMC article.
-
GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model.Int J Mol Sci. 2025 Aug 7;26(15):7634. doi: 10.3390/ijms26157634. Int J Mol Sci. 2025. PMID: 40806762 Free PMC article.
-
Obatoclax Rescues FUS-ALS Phenotypes in iPSC-Derived Neurons by Inducing Autophagy.Cells. 2023 Sep 11;12(18):2247. doi: 10.3390/cells12182247. Cells. 2023. PMID: 37759469 Free PMC article.
-
GM1 ganglioside exerts protective effects against glutamate-excitotoxicity via its oligosaccharide in wild-type and amyotrophic lateral sclerosis motor neurons.FEBS Open Bio. 2023 Dec;13(12):2324-2341. doi: 10.1002/2211-5463.13727. Epub 2023 Nov 15. FEBS Open Bio. 2023. PMID: 37885330 Free PMC article.
References
-
- Toomey C.E., Heywood W.E., Evans J.R., Lachica J., Pressey S.N., Foti S.C., Al Shahrani M., D’Sa K., Hargreaves I.P., Heales S., et al. Mitochondrial dysfunction is a key pathological driver of early stage Parkinson’s. Acta Neuropathol. Commun. 2022;10:134. doi: 10.1186/s40478-022-01424-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

