Augmenting the Angiogenic Profile and Functionality of Cord Blood Endothelial Colony-Forming Cells by Indirect Priming with Bone-Marrow-Derived Mesenchymal Stromal Cells
- PMID: 37239042
- PMCID: PMC10216643
- DOI: 10.3390/biomedicines11051372
Augmenting the Angiogenic Profile and Functionality of Cord Blood Endothelial Colony-Forming Cells by Indirect Priming with Bone-Marrow-Derived Mesenchymal Stromal Cells
Abstract
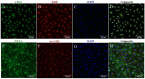
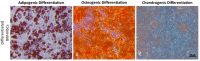
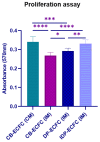
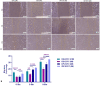
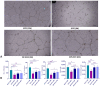
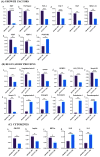
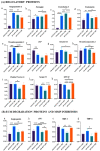
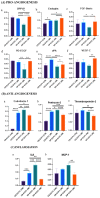
Cellular therapy has shown promise as a strategy for the functional restoration of ischemic tissues through promoting vasculogenesis. Therapy with endothelial progenitor cells (EPCs) has shown encouraging results in preclinical studies, but the limited engraftment, inefficient migration, and poor survival of patrolling endothelial progenitor cells at the injured site hinder its clinical utilization. These limitations can, to some extent, be overcome by co-culturing EPCs with mesenchymal stem cells (MSCs). Studies on the improvement in functional capacity of late EPCs, also referred to as endothelial colony-forming cells (ECFCs), when cultured with MSCs have mostly focused on the angiogenic potential, although migration, adhesion, and proliferation potential also determine effective physiological vasculogenesis. Alteration in angiogenic proteins with co-culturing has also not been studied. We co-cultured ECFCs with MSCs via both direct and indirect means, and studied the impact of the resultant contact-mediated and paracrine-mediated impact of MSCs over ECFCs, respectively, on the functional aspects and the angiogenic protein signature of ECFCs. Both directly and indirectly primed ECFCs significantly restored the adhesion and vasculogenic potential of impaired ECFCs, whereas indirectly primed ECFCs showed better proliferation and migratory potential than directly primed ECFCs. Additionally, indirectly primed ECFCs, in their angiogenesis proteomic signature, showed alleviated inflammation, along with the balanced expression of various growth factors and regulators of angiogenesis.
Keywords: bone-marrow-derived mesenchymal stem/stromal cells (BM-MSCs); co-culture; direct priming; endothelial colony-forming cells (ECFCs); functionality of ECFCs; indirect priming; proteome profiling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Contributions of Risk Factors and Medical Care to Cardiovascular Mortality Trends-PubMed. [(accessed on 30 March 2023)]; Available online: https://pubmed.ncbi.nlm.nih.gov/26076950/
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Cardiovascular Diseases (CVDs) [(accessed on 4 February 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
-
- Deaths Due to Heart Attacks up by 53% in 5 Years: NCRB|India News-Times of India. [(accessed on 4 February 2023)]. Available online: https://timesofindia.indiatimes.com/india/deaths-due-to-heart-attacks-up....
Grants and funding
LinkOut - more resources
Full Text Sources

